PET/MRI for staging patients with Hodgkin lymphoma: equivalent results with PET/CT in a prospective trial
- PMID: 33909101
- PMCID: PMC8116299
- DOI: 10.1007/s00277-021-04537-5
PET/MRI for staging patients with Hodgkin lymphoma: equivalent results with PET/CT in a prospective trial
Abstract
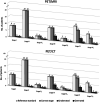
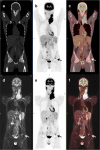
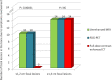
To compare FDG-PET/unenhanced MRI and FDG-PET/diagnostic CT in detecting infiltration in patients with newly diagnosed Hodgkin lymphoma (HL). The endpoint was equivalence between PET/MRI and PET/CT in correctly defining the revised Ann Arbor staging system. Seventy consecutive patients with classical-HL were prospectively investigated for nodal and extra-nodal involvement during pretreatment staging with same-day PET/CT and PET/MRI. Findings indicative of malignancy with the imaging procedures were regarded as lymphoma infiltration; in case of discrepancy, positive-biopsy and/or response to treatment were evidenced as lymphoma. Sixty of the 70 (86%) patients were evaluable having completed the staging program. Disease staging based on either PET/MRI or PET/CT was correct for 54 of the 60 patients (90% vs. 90%), with difference between proportions of 0.0 (95% CI, -9 to 9%; P=0.034 for the equivalence test). As compared with reference standard, invasion of lymph nodes was identified with PET/MRI in 100% and with PET/CT in 100%, of the spleen with PET/MRI in 66% and PET/CT in 55%, of the lung with PET/MRI in 60% and PET/CT in 100%, of the liver with PET/MRI in 67% and PET/CT in 100%, and of the bone with PET/MRI in 100% and PET/CT in 50%. The only statistically significant difference between PET/MRI and PET/CT was observed in bony infiltration detection rates. For PET/CT, iodinate contrast medium infusions' average was 86 mL, and exposure to ionizing radiation was estimated to be 4-fold higher than PET/MRI. PET/MRI is a promising safe new alternative in the care of patients with HL.
Keywords: Hodgkin lymphoma; PET/CT; PET/MRI.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Eichenauer DA, Aleman BMP, Andre M, Federico M, Hutchings M, Illidge T, Engert A, Ladetto M, clinicalguidelines@esmo.org EGCEa. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29 Suppl 4:iv19-iv29. 10.1093/annonc/mdy080 - PubMed
-
- Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Armand P, Bello CM, Benitez CM, Bierman PJ, Boughan KM, Dabaja B, Gordon LI, Hernandez-Ilizaliturri FJ, Herrera AF, Hochberg EP, Huang J, Johnston PB, Kaminski MS, Kenkre VP, Khan N, Lynch RC, Maddocks K, McConathy J, McKinney M, Metzger M, Morgan D, Mulroney C, Rabinovitch R, Rosenspire KC, Seropian S, Tao R, Winter JN, Yahalom J, Burns JL, Ogba N, Hodgkin lymphoma NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2020;18(6):755–781. doi: 10.6004/jnccn.2020.0026. - DOI - PubMed
-
- Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, Hoekstra OS, Hicks RJ, O’Doherty MJ, Hustinx R, Biggi A, Cheson BD. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048–3058. doi: 10.1200/JCO.2013.53.5229. - DOI - PMC - PubMed
-
- Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, Alliance AL, Lymphoma G, Eastern Cooperative Oncology G. European Mantle Cell Lymphoma C. Italian Lymphoma F. European Organisation for R. Treatment of Cancer/Dutch Hemato-Oncology G. Grupo Espanol de Medula O. German High-Grade Lymphoma Study G. German Hodgkin’s Study G. Japanese Lymphorra Study G. Lymphoma Study A. Group NCT. Nordic Lymphoma Study G. Southwest Oncology G. United Kingdom National Cancer Research I Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

