Cannabidiol reduces withdrawal symptoms in nicotine-dependent rats
- PMID: 33909102
- PMCID: PMC8295227
- DOI: 10.1007/s00213-021-05845-4
Cannabidiol reduces withdrawal symptoms in nicotine-dependent rats
Abstract
Rationale: Cannabidiol (CBD) reduces craving in animal models of alcohol and cocaine use and is known to modulate nicotinic receptor function, suggesting that it may alleviate symptoms of nicotine withdrawal. However, preclinical evaluation of its efficacy is still lacking.
Objectives: The goal of this study was to test the preclinical efficacy of a chronic CBD treatment in reducing nicotine dependence using measures of withdrawal symptoms including somatic signs, hyperalgesia, and weight gain during acute and protracted abstinence.
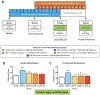
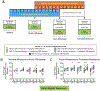
Methods: Male and female Wistar rats were made dependent on nicotine using osmotic minipumps (3.15 mg/kg/day) for 2 weeks, after which minipumps were removed to induce spontaneous withdrawal. Three groups received CBD injections at doses of 7.5, 15, and 30 mg/kg/day for 2 weeks, starting 1 week into chronic nicotine infusion. The control groups included rats with nicotine minipumps that received vehicle injections of sesame oil instead of CBD; rats implanted with saline minipumps received sesame oil injections (double vehicle) or the highest dose of CBD 30 mg/kg/day. Throughout the experiment, serum was collected for determination of CBD and nicotine concentrations, mechanical sensitivity threshold and withdrawal scores were measured, and body weight was recorded.
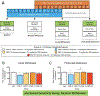
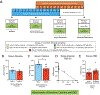
Results: CBD prevented rats from exhibiting somatic signs of withdrawal and hyperalgesia during acute and protracted abstinence. There was no dose-response observed for CBD, suggesting a ceiling effect at the doses used and the potential for lower effective doses of CBD. The saline minipump group did not show either somatic signs of withdrawal or hyperalgesia during acute and protracted abstinence, and the highest dose of CBD used (30 mg/kg/day) did not alter these results.
Conclusions: This preclinical study suggests that using CBD as a strategy to alleviate the withdrawal symptoms upon nicotine cessation may be beneficial.
Keywords: Abstinence; Addiction; CBD; Tobacco; Treatment; Withdrawal.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Figures

Similar articles
-
The effects of chronic versus acute desipramine on nicotine withdrawal and nicotine self-administration in the rat.Psychopharmacology (Berl). 2008 Jun;198(3):351-62. doi: 10.1007/s00213-008-1144-5. Epub 2008 Apr 29. Psychopharmacology (Berl). 2008. PMID: 18438738 Free PMC article.
-
Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats.Psychopharmacology (Berl). 2004 Apr;173(1-2):64-72. doi: 10.1007/s00213-003-1692-7. Epub 2004 Jan 8. Psychopharmacology (Berl). 2004. PMID: 14712336
-
Examining the clinical efficacy of bupropion and nortriptyline as smoking cessation agents in a rodent model of nicotine withdrawal.Psychopharmacology (Berl). 2007 Dec;195(3):303-13. doi: 10.1007/s00213-007-0902-0. Epub 2007 Aug 10. Psychopharmacology (Berl). 2007. PMID: 17690868
-
Rodent models for nicotine withdrawal.J Psychopharmacol. 2021 Oct;35(10):1169-1187. doi: 10.1177/02698811211005629. Epub 2021 Apr 22. J Psychopharmacol. 2021. PMID: 33888006 Free PMC article. Review.
-
Bupropion for the treatment of nicotine withdrawal and craving.Expert Rev Neurother. 2006 Jul;6(7):965-81. doi: 10.1586/14737175.6.7.965. Expert Rev Neurother. 2006. PMID: 16831112 Review.
Cited by
-
Unveiling the Potential of Phytocannabinoids: Exploring Marijuana's Lesser-Known Constituents for Neurological Disorders.Biomolecules. 2024 Oct 13;14(10):1296. doi: 10.3390/biom14101296. Biomolecules. 2024. PMID: 39456229 Free PMC article. Review.
-
The role of miRNA-144-3p/Oprk1/KOR in nicotine dependence and nicotine withdrawal in male rats.Nicotine Tob Res. 2023 Nov 22;25(12):1856-1864. doi: 10.1093/ntr/ntad118. Nicotine Tob Res. 2023. PMID: 37455648 Free PMC article.
-
New Insights in the Involvement of the Endocannabinoid System and Natural Cannabinoids in Nicotine Dependence.Int J Mol Sci. 2021 Dec 10;22(24):13316. doi: 10.3390/ijms222413316. Int J Mol Sci. 2021. PMID: 34948106 Free PMC article. Review.
-
Directed evolution unlocks oxygen reactivity for a nicotine-degrading flavoenzyme.Nat Chem Biol. 2023 Nov;19(11):1406-1414. doi: 10.1038/s41589-023-01426-y. Epub 2023 Sep 28. Nat Chem Biol. 2023. PMID: 37770699 Free PMC article.
-
Cannabidiol as a potential cessation therapeutic: Effects on intravenous nicotine self-administration and withdrawal symptoms in mice.Neuropharmacology. 2024 Mar 15;246:109833. doi: 10.1016/j.neuropharm.2023.109833. Epub 2024 Jan 3. Neuropharmacology. 2024. PMID: 38176534 Free PMC article.
References
-
- (2018). “Cannabidiol (Epidiolex) for epilepsy.” Med Lett Drugs Ther 60(1559): 182–184. - PubMed
-
- Alvarez FJ, Lafuente H, Rey-Santano MC, Mielgo VE, Gastiasoro E, Rueda M, Pertwee RG, Castillo AI, Romero J and Martinez-Orgado J (2008). “Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets.” Pediatr Res 64(6): 653–658. - PubMed
-
- Anderson GD and Chan LN (2016). “Pharmacokinetic Drug Interactions with Tobacco, Cannabinoids and Smoking Cessation Products.” Clin Pharmacokinet 55(11): 1353–1368. - PubMed
-
- Carstens E, Anderson KA, Simons CT, Carstens MI and Jinks SL (2001). “Analgesia induced by chronic nicotine infusion in rats: differences by gender and pain test.” Psychopharmacology (Berl) 157(1): 40–45. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

