How Zebrafish Can Drive the Future of Genetic-based Hearing and Balance Research
- PMID: 33909162
- PMCID: PMC8110678
- DOI: 10.1007/s10162-021-00798-z
How Zebrafish Can Drive the Future of Genetic-based Hearing and Balance Research
Abstract
Over the last several decades, studies in humans and animal models have successfully identified numerous molecules required for hearing and balance. Many of these studies relied on unbiased forward genetic screens based on behavior or morphology to identify these molecules. Alongside forward genetic screens, reverse genetics has further driven the exploration of candidate molecules. This review provides an overview of the genetic studies that have established zebrafish as a genetic model for hearing and balance research. Further, we discuss how the unique advantages of zebrafish can be leveraged in future genetic studies. We explore strategies to design novel forward genetic screens based on morphological alterations using transgenic lines or behavioral changes following mechanical or acoustic damage. We also outline how recent advances in CRISPR-Cas9 can be applied to perform reverse genetic screens to validate large sequencing datasets. Overall, this review describes how future genetic studies in zebrafish can continue to advance our understanding of inherited and acquired hearing and balance disorders.
Keywords: genetic screening; genetics; hearing and balance; zebrafish.
Conflict of interest statement
The authors declare no competing interests.
Figures
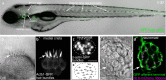

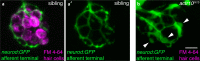


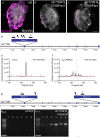

References
-
- Aman A, Nguyen M, Piotrowski T. Wnt/β-catenin dependent cell proliferation underlies segmented lateral line morphogenesis. Dev Biol. 2011;349:470–482. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

