Burn-induced hypermetabolism and skeletal muscle dysfunction
- PMID: 33909503
- PMCID: PMC8321793
- DOI: 10.1152/ajpcell.00106.2021
Burn-induced hypermetabolism and skeletal muscle dysfunction
Abstract
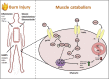
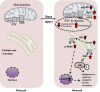
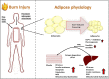
Critical illnesses, including sepsis, cancer cachexia, and burn injury, invoke a milieu of systemic metabolic and inflammatory derangements that ultimately results in increased energy expenditure leading to fat and lean mass catabolism. Burn injuries present a unique clinical challenge given the magnitude and duration of the hypermetabolic response compared with other forms of critical illness, which drastically increase the risk of morbidity and mortality. Skeletal muscle metabolism is particularly altered as a consequence of burn-induced hypermetabolism, as it primarily provides a main source of fuel in support of wound healing. Interestingly, muscle catabolism is sustained long after the wound has healed, indicating that additional mechanisms beyond wound healing are involved. In this review, we discuss the distinctive pathophysiological response to burn injury with a focus on skeletal muscle function and metabolism. We first examine the diverse consequences on skeletal muscle dysfunction between thermal, electrical, and chemical burns. We then provide a comprehensive overview of the known mechanisms underlying skeletal muscle dysfunction that may be attributed to hypermetabolism. Finally, we review the most promising current treatment options to mitigate muscle catabolism, and by extension improve morbidity and mortality, and end with future directions that have the potential to significantly improve patient care.
Keywords: inflammation; burn injury; hypermetabolism; muscle dysfunction.
Figures
References
-
- Mortality GBD and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385: 117–171, 2015. doi:10.1016/S0140-6736(14)61682-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

