Endothelial Zeb2 preserves the hepatic angioarchitecture and protects against liver fibrosis
- PMID: 33909875
- PMCID: PMC8953454
- DOI: 10.1093/cvr/cvab148
Endothelial Zeb2 preserves the hepatic angioarchitecture and protects against liver fibrosis
Abstract
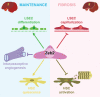
Aims: Hepatic capillaries are lined with specialized liver sinusoidal endothelial cells (LSECs) which support macromolecule passage to hepatocytes and prevent fibrosis by keeping hepatic stellate cells (HSCs) quiescent. LSEC specialization is co-determined by transcription factors. The zinc-finger E-box-binding homeobox (Zeb)2 transcription factor is enriched in LSECs. Here, we aimed to elucidate the endothelium-specific role of Zeb2 during maintenance of the liver and in liver fibrosis.
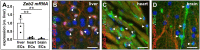
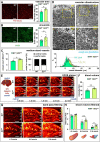
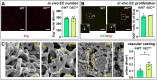
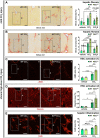
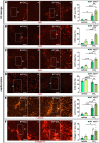
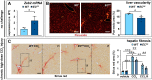
Methods and results: To study the role of Zeb2 in liver endothelium we generated EC-specific Zeb2 knock-out (ECKO) mice. Sequencing of liver EC RNA revealed that deficiency of Zeb2 results in prominent expression changes in angiogenesis-related genes. Accordingly, the vascular area was expanded and the presence of pillars inside ECKO liver vessels indicated that this was likely due to increased intussusceptive angiogenesis. LSEC marker expression was not profoundly affected and fenestrations were preserved upon Zeb2 deficiency. However, an increase in continuous EC markers suggested that Zeb2-deficient LSECs are more prone to dedifferentiation, a process called 'capillarization'. Changes in the endothelial expression of ligands that may be involved in HSC quiescence together with significant changes in the expression profile of HSCs showed that Zeb2 regulates LSEC-HSC communication and HSC activation. Accordingly, upon exposure to the hepatotoxin carbon tetrachloride (CCl4), livers of ECKO mice showed increased capillarization, HSC activation, and fibrosis compared to livers from wild-type littermates. The vascular maintenance and anti-fibrotic role of endothelial Zeb2 was confirmed in mice with EC-specific overexpression of Zeb2, as the latter resulted in reduced vascularity and attenuated CCl4-induced liver fibrosis.
Conclusion: Endothelial Zeb2 preserves liver angioarchitecture and protects against liver fibrosis. Zeb2 and Zeb2-dependent genes in liver ECs may be exploited to design novel therapeutic strategies to attenuate hepatic fibrosis.
Keywords: Capillarization; Intussusceptive angiogenesis; Liver fibrosis; Liver sinusoidal endothelial cells; Zeb2.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

References
-
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 2007;100:174–190. - PubMed
-
- Coppiello G, Collantes M, Sirerol-Piquer MS, Vandenwijngaert S, Schoors S, Swinnen M, Vandersmissen I, Herijgers P, Topal B, van Loon J, Goffin J, Prósper F, Carmeliet P, García-Verdugo JM, Janssens S, Peñuelas I, Aranguren XL, Luttun A. Meox2/Tcf15 heterodimers program the heart capillary endothelium for cardiac fatty acid uptake. Circulation 2015;131:815–826. - PubMed
-
- Geraud C, Koch PS, Zierow J, Klapproth K, Busch K, Olsavszky V, Leibing T, Demory A, Ulbrich F, Diett M, Singh S, Sticht C, Breitkopf-Heinlein K, Richter K, Karppinen SM, Pihlajaniemi T, Arnold B, Rodewald HR, Augustin HG, Schledzewski K, Goerdt S. GATA4-dependent organ-specific endothelial differentiation controls liver development and embryonic hematopoiesis. J Clin Invest 2017;127:1099–1114. - PMC - PubMed
-
- Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, Rautou PE. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol 2017;66:212–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

