Age-dependent instability of mature neuronal fate in induced neurons from Alzheimer's patients
- PMID: 33910058
- PMCID: PMC8423435
- DOI: 10.1016/j.stem.2021.04.004
Age-dependent instability of mature neuronal fate in induced neurons from Alzheimer's patients
Abstract
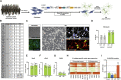
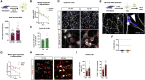
Sporadic Alzheimer's disease (AD) exclusively affects elderly people. Using direct conversion of AD patient fibroblasts into induced neurons (iNs), we generated an age-equivalent neuronal model. AD patient-derived iNs exhibit strong neuronal transcriptome signatures characterized by downregulation of mature neuronal properties and upregulation of immature and progenitor-like signaling pathways. Mapping iNs to longitudinal neuronal differentiation trajectory data demonstrated that AD iNs reflect a hypo-mature neuronal identity characterized by markers of stress, cell cycle, and de-differentiation. Epigenetic landscape profiling revealed an underlying aberrant neuronal state that shares similarities with malignant transformation and age-dependent epigenetic erosion. To probe for the involvement of aging, we generated rejuvenated iPSC-derived neurons that showed no significant disease-related transcriptome signatures, a feature that is consistent with epigenetic clock and brain ontogenesis mapping, which indicate that fibroblast-derived iNs more closely reflect old adult brain stages. Our findings identify AD-related neuronal changes as age-dependent cellular programs that impair neuronal identity.
Keywords: Alzheimer's disease; aging; de-differentiation; induced neurons (iNs); neuronal cell cycle re-entry; rejuvenation.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests F.H.G. is an advisory board member of Cell Stem Cell.
Figures






References
-
- Alzheimer’s Association 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11:332–384. - PubMed
-
- Andreadis A. Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim. Biophys. Acta Bba - Mol Basis Dis. 2005;1739:91–103. - PubMed
-
- Annese A., Manzari C., Lionetti C., Picardi E., Horner D.S., Chiara M., Caratozzolo M.F., Tullo A., Fosso B., Pesole G., D’Erchia A.M. Whole transcriptome profiling of Late-Onset Alzheimer’s Disease patients provides insights into the molecular changes involved in the disease. Sci. Rep. 2018;8:4282. - PMC - PubMed
-
- Arbab M., Baars S., Geijsen N. Modeling motor neuron disease: the matter of time. Trends Neurosci. 2014;37:642–652. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

