PEGylated nanoparticle albumin-bound steroidal ginsenoside derivatives ameliorate SARS-CoV-2-mediated hyper-inflammatory responses
- PMID: 33910079
- PMCID: PMC8046382
- DOI: 10.1016/j.biomaterials.2021.120827
PEGylated nanoparticle albumin-bound steroidal ginsenoside derivatives ameliorate SARS-CoV-2-mediated hyper-inflammatory responses
Abstract
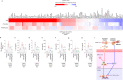
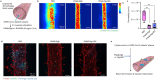
The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on a global scale urges prompt and effective countermeasures. Recently, a study has reported that coronavirus disease-19 (COVID-19), the disease caused by SARS-CoV-2 infection, is associated with a decrease in albumin level, an increase in NETosis, blood coagulation, and cytokine level. Here, we present drug-loaded albumin nanoparticles as a therapeutic agent to resolve the clinical outcomes observed in severe SARS-CoV-2 patients. PEGylated nanoparticle albumin-bound (PNAB) was used to promote prolonged bioactivity of steroidal ginsenoside saponins, PNAB-Rg6 and PNAB-Rgx365. Our data indicate that the application of PNAB-steroidal ginsenoside can effectively reduce histone H4 and NETosis-related factors in the plasma, and alleviate SREBP2-mediated systemic inflammation in the PBMCs of SARS-CoV-2 ICU patients. The engineered blood vessel model confirmed that these drugs are effective in suppressing blood clot formation and vascular inflammation. Moreover, the animal model experiment showed that these drugs are effective in promoting the survival rate by alleviating tissue damage and cytokine storm. Altogether, our findings suggest that these PNAB-steroidal ginsenoside drugs have potential applications in the treatment of symptoms associated with severe SARS-CoV-2 patients, such as coagulation and cytokine storm.
Keywords: Albumin; COVID-19; Cytokine storm; Ginsenoside; NETosis.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

