Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage Into Cord Blood
- PMID: 33910219
- PMCID: PMC8288193
- DOI: 10.1097/AOG.0000000000004438
Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage Into Cord Blood
Abstract
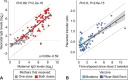
Coronavirus disease 2019 (COVID-19) vaccination in pregnancy induces a robust maternal immune response, with transplacental antibody transfer detectable in cord blood as early as 16 days after the first dose.
Conflict of interest statement
Financial Disclosure Zhen Zhao received seed instruments and sponsored research from ET Healthcare and sponsored research from Roche. The other authors did not report any potential conflicts of interest.
Figures

Comment on
-
Patient care and clinical outcomes for patients with COVID-19 infection admitted to African high-care or intensive care units (ACCCOS): a multicentre, prospective, observational cohort study.Lancet. 2021 May 22;397(10288):1885-1894. doi: 10.1016/S0140-6736(21)00441-4. Lancet. 2021. PMID: 34022988 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

