Maternal Antibody Response, Neutralizing Potency, and Placental Antibody Transfer After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection
- PMID: 33910220
- PMCID: PMC8288196
- DOI: 10.1097/AOG.0000000000004440
Maternal Antibody Response, Neutralizing Potency, and Placental Antibody Transfer After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection
Abstract
Objective: To characterize maternal immune response after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during pregnancy and quantify the efficiency of transplacental antibody transfer.
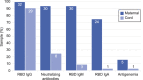
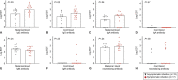
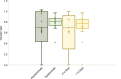
Methods: We conducted a prospective cohort study of pregnant patients who tested positive for SARS CoV-2 infection at any point in pregnancy and collected paired maternal and cord blood samples at the time of delivery. An enzyme-linked immunosorbent assay (ELISA) and neutralization assays were performed to measure maternal plasma and cord blood concentrations and neutralizing potency of immunoglobulin (Ig)G, IgA, and IgM antibodies directed against the SARS-CoV-2 spike protein. Differences in concentrations according to symptomatic compared with asymptomatic infection and time from positive polymerase chain reaction (PCR) test result to delivery were analyzed using nonparametric tests of significance. The ratio of cord to maternal anti-receptor-binding domain IgG titers was analyzed to assess transplacental transfer efficiency.
Results: Thirty-two paired samples were analyzed. Detectable anti-receptor-binding domain IgG was detected in 100% (n=32) of maternal and 91% (n=29) of cord blood samples. Functional neutralizing antibody was present in 94% (n=30) of the maternal and 25% (n=8) of cord blood samples. Symptomatic infection was associated with a significant difference in median (interquartile range) maternal anti-receptor-binding domain IgG titers compared with asymptomatic infection (log 3.2 [3.5-2.4] vs log 2.7 [2.9-1.4], P=.03). Median (interquartile range) maternal anti-receptor-binding domain IgG titers were not significantly higher in patients who delivered more than 14 days after a positive PCR test result compared with those who delivered within 14 days (log 3.3 [3.5-2.4] vs log 2.67 [2.8-1.6], P=.05). Median (range) cord/maternal antibody ratio was 0.81 (0.67-0.88).
Conclusions: These results demonstrate robust maternal neutralizing and anti-receptor-binding domain IgG response after SARS-CoV-2 infection, yet a lower-than-expected efficiency of transplacental antibody transfer and a significant reduction in neutralization between maternal blood and cord blood. Maternal infection does confer some degree of neonatal antibody protection, but the robustness and durability of protection require further study.
Copyright © 2021 by the American College of Obstetricians and Gynecologists. Published by Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
Financial Disclosure The authors did not report any potential conflicts of interest.
Figures

References
-
- Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. . Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1641–7. doi: 10.15585/mmwr.mm6944e3 - DOI - PMC - PubMed
-
- Kim L, Whitaker M, O’Halloran A, Kambhampati A, Chai S, Reingold A, et al. . Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19-COVID-NET, 14 states, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1081–8. doi: 10.15585/mmwr.mm6932e3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

