The role of Sphingomyelin synthase 2 (SMS2) in platelet activation and its clinical significance
- PMID: 33910580
- PMCID: PMC8082820
- DOI: 10.1186/s12959-021-00282-x
The role of Sphingomyelin synthase 2 (SMS2) in platelet activation and its clinical significance
Abstract
Background: Sphingomyelin (SM) is an essential component of biological lipid rafts, and it plays an indispensable role in maintaining plasma membrane stability and in mediating signal transduction. The ultimate biosynthesis of SM is catalyzed by two sphingomyelin synthases (SMSs) namely SMS1 and SMS2, which are selectively distributed in the trans-Golgi apparatus and the plasma membrane. It has been demonstrated that SMS2 acts as an irreplaceable molecule in the regulation of transmembrane signaling, and loss of SMS2 has been reported to worsen atherosclerosis and liver steatosis. However, the function of SMS2 in platelet activation and its association with the pathological process of thrombosis in acute coronary syndrome (ACS) and portal hypertension (PH) remain unclear.
Methods: In this study, we tested the role of SMS2 in platelet activation and thrombosis using SMS2 knockout (SMS2 -/-) mice and SMS2-specific inhibitor, D609. Furthermore, we detected SMS2 expression in patients with ACS and PH.
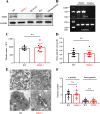
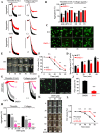
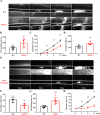
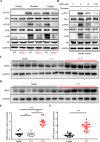
Results: SMS2 -/- platelets showed significant reduction in platelet aggregation, spreading, clot retraction and in vivo thrombosis. Similar inhibitory effects on platelet activation were detected in D609-treated wild-type platelets. PLCγ/PI3K/Akt signaling pathway was inhibited in SMS2 -/- platelets and D609-treated wild-type platelets. In addition, we discovered that platelet SMS2 expression was remarkably increased in patients with ACS and PH, compared with healthy subjects.
Conclusions: Our study indicates that SMS2 acts as a positive regulator of platelet activation and thrombosis, and provides a theoretical basis for the potential use of D609 in anti-thrombosis treatment.
Keywords: D609; Platelet; SMS2; Thrombosis.
Conflict of interest statement
The authors declare that they have no competing interests” in this section.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

