Charge transport physics of a unique class of rigid-rod conjugated polymers with fused-ring conjugated units linked by double carbon-carbon bonds
- PMID: 33910909
- PMCID: PMC8081371
- DOI: 10.1126/sciadv.abe5280
Charge transport physics of a unique class of rigid-rod conjugated polymers with fused-ring conjugated units linked by double carbon-carbon bonds
Abstract
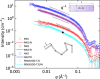
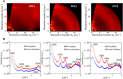
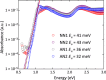
We investigate the charge transport physics of a previously unidentified class of electron-deficient conjugated polymers that do not contain any single bonds linking monomer units along the backbone but only double-bond linkages. Such polymers would be expected to behave as rigid rods, but little is known about their actual chain conformations and electronic structure. Here, we present a detailed study of the structural and charge transport properties of a family of four such polymers. By adopting a copolymer design, we achieve high electron mobilities up to 0.5 cm2 V-1 s-1 Field-induced electron spin resonance measurements of charge dynamics provide evidence for relatively slow hopping over, however, long distances. Our work provides important insights into the factors that limit charge transport in this unique class of polymers and allows us to identify molecular design strategies for achieving even higher levels of performance.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Bao Z., Dodabalapur A., Lovinger A. J., Soluble and processable regioregular poly(3-hexylthiophene) for thin film field-effect transistor applications with high mobility. Appl. Phys. Lett. 69, 4108–4110 (1996).
-
- Burroughes J. H., Bradley D. D. C., Brown A. R., Marks R. N., Mackay K., Friend R. H., Burns P. L., Holmes A. B., Light-emitting diodes based on conjugated polymers. Nature 347, 539–541 (1990).
-
- Arias A. C., MacKenzie J. D., McCulloch I., Rivnay J., Salleo A., Materials and applications for large area electronics: Solution-based approaches. Chem. Rev. 110, 3–24 (2010). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

