Cancer Cells Retrace a Stepwise Differentiation Program during Malignant Progression
- PMID: 33910926
- PMCID: PMC7611766
- DOI: 10.1158/2159-8290.CD-20-1637
Cancer Cells Retrace a Stepwise Differentiation Program during Malignant Progression
Abstract
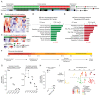
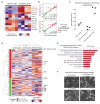
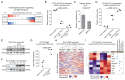
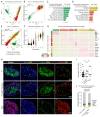
Pancreatic neuroendocrine tumors (PanNET) comprise two molecular subtypes, relatively benign islet tumors (IT) and invasive, metastasis-like primary (MLP) tumors. Until now, the origin of aggressive MLP tumors has been obscure. Herein, using multi-omics approaches, we revealed that MLP tumors arise from IT via dedifferentiation following a reverse trajectory along the developmental pathway of islet β cells, which results in the acquisition of a progenitor-like molecular phenotype. Functionally, the miR-181cd cluster induces the IT-to-MLP transition by suppressing expression of the Meis2 transcription factor, leading to upregulation of a developmental transcription factor, Hmgb3. Notably, the IT-to-MLP transition constitutes a distinct step of tumorigenesis and is separable from the classic proliferation-associated hallmark, temporally preceding accelerated proliferation of cancer cells. Furthermore, patients with PanNET with elevated HMGB3 expression and an MLP transcriptional signature are associated with higher-grade tumors and worse survival. Overall, our results unveil a new mechanism that modulates cancer cell plasticity to enable malignant progression. SIGNIFICANCE: Dedifferentiation has long been observed as a histopathologic characteristic of many cancers, albeit inseparable from concurrent increases in cell proliferation. Herein, we demonstrate that dedifferentiation is a mechanistically and temporally separable step in the multistage tumorigenesis of pancreatic islet cells, retracing the developmental lineage of islet β cells.This article is highlighted in the In This Issue feature, p. 2355.
©2021 American Association for Cancer Research.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Zhou C, Zhang J, Zheng Y, Zhu Z. Pancreatic neuroendocrine tumors: A comprehensive review. Int J Cancer. 2012;131:1013–22. - PubMed
-
- Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770–86. - PMC - PubMed
-
- Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543:65–71. - PubMed
-
- Hanahan D. Heritable formation of pancreatic β-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

