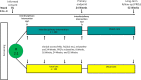
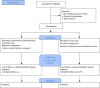
Effectiveness of interdisciplinary combined dermatology-gastroenterology-rheumatology clinical care compared to usual care in patients with immune-mediated inflammatory diseases: a parallel group, non-blinded, pragmatic randomised trial
- PMID: 33910945
- PMCID: PMC8094387
- DOI: 10.1136/bmjopen-2020-041871
Effectiveness of interdisciplinary combined dermatology-gastroenterology-rheumatology clinical care compared to usual care in patients with immune-mediated inflammatory diseases: a parallel group, non-blinded, pragmatic randomised trial
Abstract
Introduction: Immune-mediated inflammatory diseases (IMIDs) are associated with reduced health-related quality of life (HRQol), increased risk of somatic and psychiatric comorbidities and reduced socioeconomic status. Individuals with one IMID have an increased risk for developing other IMIDs. The unmet needs in the care of patients with IMIDs may result from a lack of patient-centricity in the usual monodisciplinary siloed approach to these diseases. The advantages of novel interdisciplinary clinics towards the traditional therapeutic approach have not been investigated. The overall aim of this study is to determine the effectiveness of an interdisciplinary combined clinic intervention compared with usual care in a population of patients with the IMIDs: psoriasis, hidradenitis suppurativa, psoriatic arthritis, axial spondyloarthritis and inflammatory bowel disease. Our hypothesis is that an interdisciplinary combined clinic intervention will be more effective than usual care in improving clinical and patient-reported outcomes, and that a more effective screening and management of other IMIDs and comorbidities can be performed.
Methods and analysis: This is a randomised, usual care controlled, parallel-group pragmatic clinical trial. 300 consecutively enrolled participants with co-occurrence of at least two IMIDs are randomly assigned in a 2:1 ratio to either treatment in the interdisciplinary combined clinic or usual care. The study will consist of a 6-month active intervention period and a 6-month follow-up period where no intervention or incentives will be provided by the trial. The primary outcome is the change from baseline to 24 weeks on the Short-Form Health Survey (SF-36) Physical Component Summary. Additional patient-reported outcome measures and clinical measures are assessed as secondary outcomes.
Ethics and dissemination: Ethical approval of this study protocol was established by the institutional review board of the study site. The findings from this trial will be disseminated via conference presentations and publications in peer-reviewed journals, and by engagement with patient organisations.
Trial registration number: NCT04200690.
Keywords: immunology; inflammatory bowel disease; organisation of health services; psoriasis; rheumatology; therapeutics.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AD has received speaking fees from Pfizer. AGL has been a consultant and advisor for the following companies: AbbVie, Eli Lilly, MSD, Novartis, Pfizer and UCB and has received speaking fees from: AbbVie, MSD, Novartis, Pfizer and UCB. JA has been consultant, advisory board member or speaker for the following companies: AbbVie, MSD, Bristol Meyer Squibb, Ferring Pharmaceuticals, Pfizer, Janssen-Cilag and Takeda. KFH has been a consultant and advisor for the following companies: AbbVie, LEO Pharma, Novartis and has received speaking fees from: AbbVie, LEO Pharma, Novartis, Janssen, CSL Behring. LI has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by: AbbVie, Almirall, Amgen, Astra Zeneca, BMS, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Janssen Cilag, Kyowa, Leo Pharma, MSD, Novartis, Pfizer, Samsung, UCB. LFMø has been advisory board member for Janssen and has received speaking fees from LEO Pharma. Robin Christensen reports no conflicts of interest. TBL has been a consultant and advisor for UCB.
Figures
Similar articles
-
On the Interdisciplinary Treatment and Management of Patients with Immune-Mediated Inflammatory Diseases. A Study on Patients' Personal Experiences and Perspectives.J Multidiscip Healthc. 2024 May 27;17:2635-2646. doi: 10.2147/JMDH.S432820. eCollection 2024. J Multidiscip Healthc. 2024. PMID: 38828269 Free PMC article.
-
Bridging the Gaps in the Care of Psoriasis and Psoriatic Arthritis: the Role of Combined Clinics.Curr Rheumatol Rep. 2018 Oct 26;20(12):76. doi: 10.1007/s11926-018-0785-6. Curr Rheumatol Rep. 2018. PMID: 30367311 Review.
-
Risk of Developing Additional Immune-Mediated Manifestations: A Retrospective Matched Cohort Study.Adv Ther. 2019 Jul;36(7):1672-1683. doi: 10.1007/s12325-019-00964-z. Epub 2019 May 17. Adv Ther. 2019. PMID: 31102202 Free PMC article.
-
Digital Outcome Measurement to Improve Care for Patients With Immune-Mediated Inflammatory Diseases: Protocol for the IMID Registry.JMIR Res Protoc. 2023 Mar 30;12:e43230. doi: 10.2196/43230. JMIR Res Protoc. 2023. PMID: 36995758 Free PMC article.
-
Early Recognition and Treatment Heralds Optimal Outcomes: the Benefits of Combined Rheumatology-Dermatology Clinics and Integrative Care of Psoriasis and Psoriatic Arthritis Patients.Curr Rheumatol Rep. 2017 Nov 20;20(1):1. doi: 10.1007/s11926-017-0706-0. Curr Rheumatol Rep. 2017. PMID: 29185062 Review.
Cited by
-
Patient characteristics, treatment patterns and disease outcomes in patients with psoriatic arthritis followed in a combined Dermatology-Rheumatology clinic: a retrospective real-world study.Rheumatol Int. 2022 Jun;42(6):1035-1041. doi: 10.1007/s00296-022-05126-z. Epub 2022 Apr 18. Rheumatol Int. 2022. PMID: 35435445
-
On the Interdisciplinary Treatment and Management of Patients with Immune-Mediated Inflammatory Diseases. A Study on Patients' Personal Experiences and Perspectives.J Multidiscip Healthc. 2024 May 27;17:2635-2646. doi: 10.2147/JMDH.S432820. eCollection 2024. J Multidiscip Healthc. 2024. PMID: 38828269 Free PMC article.
-
A multidisciplinary dermatology-gastroenterology-rheumatology (DER.RE.GA) unit for the care of patients with immune-mediated inflammatory diseases: analysis of the first 5 years from the dermatologist's perspective.Front Med (Lausanne). 2023 Nov 30;10:1290018. doi: 10.3389/fmed.2023.1290018. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38098849 Free PMC article.
-
Reducing diagnostic delays of extraintestinal manifestations in inflammatory bowel disease: a comparative study of a multidisciplinary outpatient clinic versus conventional referral specialists.Therap Adv Gastroenterol. 2025 Mar 3;18:17562848251323529. doi: 10.1177/17562848251323529. eCollection 2025. Therap Adv Gastroenterol. 2025. PMID: 40041240 Free PMC article.
-
Comorbidity in Adult Psoriasis: Considerations for the Clinician.Psoriasis (Auckl). 2022 Jun 10;12:139-150. doi: 10.2147/PTT.S328572. eCollection 2022. Psoriasis (Auckl). 2022. PMID: 35712227 Free PMC article. Review.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical