SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma
- PMID: 33910979
- PMCID: PMC8362330
- DOI: 10.1126/scitranslmed.abe7378
SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma
Abstract
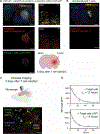
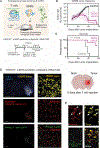
Treatment of solid cancers with chimeric antigen receptor (CAR) T cells is plagued by the lack of ideal target antigens that are both absolutely tumor specific and homogeneously expressed. We show that multi-antigen prime-and-kill recognition circuits provide flexibility and precision to overcome these challenges in the context of glioblastoma. A synNotch receptor that recognizes a specific priming antigen, such as the heterogeneous but tumor-specific glioblastoma neoantigen epidermal growth factor receptor splice variant III (EGFRvIII) or the central nervous system (CNS) tissue-specific antigen myelin oligodendrocyte glycoprotein (MOG), can be used to locally induce expression of a CAR. This enables thorough but controlled tumor cell killing by targeting antigens that are homogeneous but not absolutely tumor specific. Moreover, synNotch-regulated CAR expression averts tonic signaling and exhaustion, maintaining a higher fraction of the T cells in a naïve/stem cell memory state. In immunodeficient mice bearing intracerebral patient-derived xenografts (PDXs) with heterogeneous expression of EGFRvIII, a single intravenous infusion of EGFRvIII synNotch-CAR T cells demonstrated higher antitumor efficacy and T cell durability than conventional constitutively expressed CAR T cells, without off-tumor killing. T cells transduced with a synNotch-CAR circuit primed by the CNS-specific antigen MOG also exhibited precise and potent control of intracerebral PDX without evidence of priming outside of the brain. In summary, by using circuits that integrate recognition of multiple imperfect but complementary antigens, we improve the specificity, completeness, and persistence of T cells directed against glioblastoma, providing a general recognition strategy applicable to other solid tumors.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



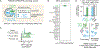
Comment in
-
New CAR's bells and whistles.Nat Rev Drug Discov. 2021 Jun;20(6):425. doi: 10.1038/d41573-021-00084-w. Nat Rev Drug Discov. 2021. PMID: 33981090 No abstract available.
-
Getting better mileage with logically primed CARs.Med. 2021 Jul 9;2(7):785-787. doi: 10.1016/j.medj.2021.06.002. Med. 2021. PMID: 35590214
References
-
- Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee C-CR, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA, Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–546 (2009). - PMC - PubMed
-
- Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan D-AN, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA, T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. J. Am. Soc. Gene Ther 19, 620–626 (2011). - PMC - PubMed
-
- Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, Phan GQ, Hughes MS, Kammula US, Miller AD, Hessman CJ, Stewart AA, Restifo NP, Quezado MM, Alimchandani M, Rosenberg AZ, Nath A, Wang T, Bielekova B, Wuest SC, Akula N, McMahon FJ, Wilde S, Mosetter B, Schendel DJ, Laurencot CM, Rosenberg SA, Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother 36, 133–151 (2013). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

