Cryptic β-Lactamase Evolution Is Driven by Low β-Lactam Concentrations
- PMID: 33910990
- PMCID: PMC8092134
- DOI: 10.1128/mSphere.00108-21
Cryptic β-Lactamase Evolution Is Driven by Low β-Lactam Concentrations
Abstract
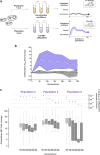
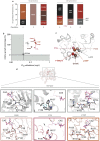
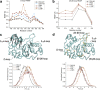
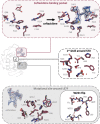
Our current understanding of how low antibiotic concentrations shape the evolution of contemporary β-lactamases is limited. Using the widespread carbapenemase OXA-48, we tested the long-standing hypothesis that selective compartments with low antibiotic concentrations cause standing genetic diversity that could act as a gateway to developing clinical resistance. Here, we subjected Escherichia coli expressing blaOXA-48, on a clinical plasmid, to experimental evolution at sub-MICs of ceftazidime. We identified and characterized seven single variants of OXA-48. Susceptibility profiles and dose-response curves showed that they increased resistance only marginally. However, in competition experiments at sub-MICs of ceftazidime, they demonstrated strong selectable fitness benefits. Increased resistance was also reflected in elevated catalytic efficiencies toward ceftazidime. These changes are likely caused by enhanced flexibility of the Ω- and β5-β6 loops and fine-tuning of preexisting active site residues. In conclusion, low-level concentrations of β-lactams can drive the evolution of β-lactamases through cryptic phenotypes which may act as stepping-stones toward clinical resistance.IMPORTANCE Very low antibiotic concentrations have been shown to drive the evolution of antimicrobial resistance. While substantial progress has been made to understand the driving role of low concentrations during resistance development for different antimicrobial classes, the importance of β-lactams, the most commonly used antibiotics, is still poorly studied. Here, we shed light on the evolutionary impact of low β-lactam concentrations on the widespread β-lactamase OXA-48. Our data indicate that the exposure to β-lactams at very low concentrations enhances β-lactamase diversity and drives the evolution of β-lactamases by significantly influencing their substrate specificity. Thus, in contrast to high concentrations, low levels of these drugs may substantially contribute to the diversification and divergent evolution of these enzymes, providing a standing genetic diversity that can be selected and mobilized when antibiotic pressure increases.
Keywords: Escherichia coli; OXA-48; carbapenem; carbapenemase; catalytic efficiency; ceftazidime; cryptic evolution; resistance development; structural flexibility; sub-MIC.
Copyright © 2021 Fröhlich et al.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
