Transcatheter mitral valve repair: an overview of current and future devices
- PMID: 33911022
- PMCID: PMC8094333
- DOI: 10.1136/openhrt-2020-001564
Transcatheter mitral valve repair: an overview of current and future devices
Erratum in
-
Correction: Transcatheter mitral valve repair: an overview of current and future devices.Open Heart. 2021 Jun;8(1):e001564corr1. doi: 10.1136/openhrt-2020-001564corr1. Open Heart. 2021. PMID: 34083393 Free PMC article. No abstract available.
Abstract
The field of transcatheter mitral valve repair (TMVr) for mitral regurgitation (MR) is rapidly evolving. Besides the well-established transcatheter mitral edge-to-edge repair approach, there is also growing evidence for therapeutic strategies targeting the mitral annulus and mitral valve chordae. A patient-tailored approach, careful patient selection and an experienced interventional team is crucial in order to optimise procedural and clinical outcomes. With further data from ongoing clinical trials to be expected, consensus in the Heart Team is needed to address these complexities and determine the most appropriate TMVr therapy, either single or combined, for patients with severe MR.
Keywords: endovascular procedures; heart valve prosthesis implantation; mitral valve insufficiency.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ODB received institutional research grants and consulting fees from Abbott and Boston Scientific. MT is a consultant for Abbott Vascular, Boston Scientific and 4tech; received personal fees from Edwards Lifesciences, Mitraltech, CoreMedic and Swissvortex; and is a shareholder of 4Tech. FM obtained grant and/or research institutional support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific Corporation, NVT, Terumo, Consulting fees, Honoraria personal and institutional from Abbott, Medtronic, Edwards Lifesciences, Xeltis, Cardiovalve, Occlufit, Simulands, Occlufit; has Royalty Income/IP Rights Edwards Lifesciences; and is shareholder (including share options) of Cardiogard, Magenta, SwissVortex, Transseptal solutions, Occlufit, 4Tech, Perifect. LS received institutional research grants and consulting fees from Abbott, Boston Scientific, Medtronic and Edwards Lifesciences. All other coauthors have no conflict of interest to disclose concerning this manuscript.
Figures

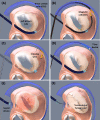
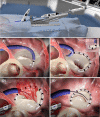
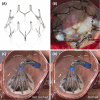

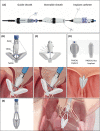

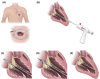
References
-
- Rogers JH, Thomas M, Morice M-C, et al. . Treatment of heart failure with associated functional mitral regurgitation using the ARTO system: initial results of the first-in-human MAVERIC trial (mitral valve repair clinical trial). JACC Cardiovasc Interv 2015;8:1095–104. 10.1016/j.jcin.2015.04.012 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
