Interleukin 10 level in the peritoneal cavity is a prognostic marker for peritoneal recurrence of T4 colorectal cancer
- PMID: 33911154
- PMCID: PMC8080840
- DOI: 10.1038/s41598-021-88653-2
Interleukin 10 level in the peritoneal cavity is a prognostic marker for peritoneal recurrence of T4 colorectal cancer
Abstract
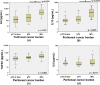
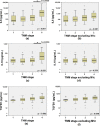
Peritoneal recurrence (PR) is a major relapse pattern of colorectal cancer (CRC). We investigated whether peritoneal immune cytokines can predict PR. Cytokine concentrations of peritoneal fluid from CRC patients were measured. Patients were grouped according to peritoneal cancer burden (PCB): no tumor cells (≤ pT3), microscopic tumor cells (pT4), or gross tumors (M1c). Cytokine concentrations were compared among the three groups and the associations of those in pT4 patients with and without postoperative PR were assessed. Of the ten cytokines assayed, IL6, IL10, and TGFB1 increased with progression of PCB. Among these, IL10 was a marker of PR in pT4 (N = 61) patients based on ROC curve (p = 0.004). The IL10 cut-off value (14 pg/mL) divided patients into groups with a low (7%, 2 of 29 patients) or high (45%, 16 of 32 patients) 5-year PR (p < 0.001). Multivariable analysis identified high IL10 levels as the independent risk factor for PR. Separation of patients into training and test sets to evaluate the performance of IL10 cut-off model validated this cytokine as a risk factor for PR. Peritoneal IL10 is a prognostic marker of PR in pT4 CRC. Further research is necessary to identify immune response of intraperitoneal CRC growth.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

