Xiaoyaosan Alleviates Hippocampal Glutamate-Induced Toxicity in the CUMS Rats via NR2B and PI3K/Akt Signaling Pathway
- PMID: 33912031
- PMCID: PMC8075411
- DOI: 10.3389/fphar.2021.586788
Xiaoyaosan Alleviates Hippocampal Glutamate-Induced Toxicity in the CUMS Rats via NR2B and PI3K/Akt Signaling Pathway
Erratum in
-
Corrigendum: Xiaoyaosan alleviates hippocampal glutamate-induced toxicity in the CUMS rats via NR2B and PI3K/Akt signaling pathway.Front Pharmacol. 2022 Dec 15;13:1042762. doi: 10.3389/fphar.2022.1042762. eCollection 2022. Front Pharmacol. 2022. PMID: 36588727 Free PMC article.
Abstract
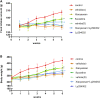
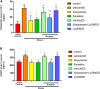
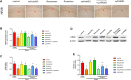
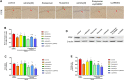
Purpose: It is revealed that Xiaoyaosan could reduce glutamate level in the hippocampus of depressed rats, whose metabolism leads to the pathophysiology of depression. However, the underlying mechanism remains unclear. This study aims to explore the effect of Xiaoyaosan on glutamate metabolism, and how to regulate the excitatory injury caused by glutamate. Methods: Rats were induced by chronic unpredictable mild stress, then divided into control, vehicle (distilled water), Xiaoyaosan, fluoxetine, vehicle (DMSO), Xiaoyaosan + Ly294002 and Ly294002 groups. Ly294002 was microinjected into the lateral ventricular catheterization at 5 mM. Xiaoyaosan (2.224 g/kg) and fluoxetine (2.0 mg/kg) were orally administered for three weeks. The open field test (OFT), forced swimming test (FST), and sucrose preference test (SPT) were used to assess depressive behavior. The glutamate and corticosterone (CORT) levels were detected by ELISA. Western blot, immunochemistry or immunofluorescence were used to detect the expressions of NR2B, MAP2, PI3K and P-AKT/Akt in the hippocampal CA1 region. The mRNA level of MAP2, NR2B and PI3K were detected by RT-qPCR. Results: Compared to the rats in control group, body weight and food intake of CUMS rats was decreased. CUMS rats also showed depression-like behavior as well as down regulate the NR2B and PI3K/Akt signaling pathway. Xiaoyaosan treatments could increase food intake and body weight as well as improved time spent in the central area, total distance traveled in the OFT. Xiaoyaosan could also decrease the immobility time as well as increase the sucrose preference in SPT. Moreover, xiaoyaosan decreased the level of glutamate in the hippocampal CA1 region and serum CORT in CUMS rats. Furthermore, xiaoyaosan improved the expression of MAP2 as well as increased the expression of NR2B, PI3K and the P-AKT/AKT ratio in the hippocampal CA1 region in the CUMS rats. Conclusion: Xiaoyaosan treatment can exert the antidepressant effect by rescuing hippocampal neurons loss induced by the glutamate-mediated excitotoxicity in CUMS rats. The underlying pathway maybe through NR2B and PI3K/Akt signaling pathways. These results may suggest the potential of Xiaoyaosan in preventing the development of depression.
Keywords: NR2B, PI3K/Akt pathway; depression; glutamate; hippocampal CA1 region; xiaoyaosan.
Copyright © 2021 Zhou, Liu, Liu, Ma, Zhao, Jiang, Li and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures



References
-
- Abdel-Rahman A., Abou-Donia S., El-Masry E., Shetty A., Abou-Donia M. (2004). Stress and combined exposure to low doses of pyridostigmine bromide, DEET, and permethrin produce neurochemical and neuropathological alterations in cerebral cortex, hippocampus, and cerebellum. J. Toxicol. Environ. Health Part A 67, 163–192. 10.1080/15287390490264802 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

