The Affinity of Hemoglobin for Oxygen Is Not Altered During COVID-19
- PMID: 33912067
- PMCID: PMC8072381
- DOI: 10.3389/fphys.2021.578708
The Affinity of Hemoglobin for Oxygen Is Not Altered During COVID-19
Abstract
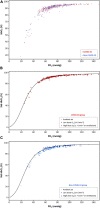
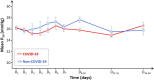
Background: A computational proteomic analysis suggested that SARS-CoV-2 might bind to hemoglobin (Hb). The authors hypothesized that this phenomenon could result in a decreased oxygen (O2) binding and lead to hemolytic anemia as well. The aim of this work was to investigate whether the affinity of Hb for O2 was altered during COVID-19. Methods: In this retrospective, observational, single-center study, the blood gas analyses of 100 COVID-19 patients were compared to those of 100 non-COVID-19 patients. Fifty-five patients with carboxyhemoglobin (HbCO) ≥8% and 30 with sickle cell disease (SCD) were also included ("positive controls" with abnormal Hb affinity). P50 was corrected for body temperature, pH, and PCO2. Results: Patients did not differ statistically for age or sex ratio in COVID-19 and non-COVID-19 groups. Median P50 at baseline was 26 mmHg [25.2-26.8] vs. 25.9 mmHg [24-27.3], respectively (p = 0.42). As expected, P50 was 22.5 mmHg [21.6-23.8] in the high HbCO group and 29.3 mmHg [27-31.5] in the SCD group (p < 0.0001). Whatever the disease severity, samples from COVID-19 to non-COVID-19 groups were distributed on the standard O2-Hb dissociation curve. When considering the time-course of P50 between days 1 and 18 in both groups, no significant difference was observed. Median Hb concentration at baseline was 14 g.dl-1 [12.6-15.2] in the COVID-19 group vs. 13.2 g.dl-1 [11.4-14.7] in the non-COVID-19 group (p = 0.006). Among the 24 COVID-19 patients displaying anemia, none of them exhibited obvious biological hemolysis. Conclusion: There was no biological argument to support the hypothesis that SARS-CoV-2 could alter O2 binding to Hb.
Keywords: COVID-19; P50; SARS-CoV-2; anemia; gas exchange; gas transport; hemoglobin-oxygen affinity; hemolysis.
Copyright © 2021 Gille, Sesé, Aubourg, Fabre, Cymbalista, Ratnam, Valeyre, Nunes, Richalet and Planès.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Afra T. P., Nampoothiri R. V., Razmi T. M., Hafi N. A. B. (2020a). Linking hydroxychloroquine to hemolysis in a ‘suspected’ glucose-6-phosphate dehydrogenase deficient patient with COVID-19 infection – a critical appraisal. Eur. J. Intern. Med. 80 101–102. 10.1016/j.ejim.2020.07.001 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

