Vasculitis and Neutrophil Extracellular Traps in Lungs of Golden Syrian Hamsters With SARS-CoV-2
- PMID: 33912167
- PMCID: PMC8072219
- DOI: 10.3389/fimmu.2021.640842
Vasculitis and Neutrophil Extracellular Traps in Lungs of Golden Syrian Hamsters With SARS-CoV-2
Abstract
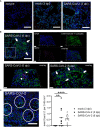
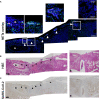
Neutrophil extracellular traps (NETs) have been identified as one pathogenetic trigger in severe COVID-19 cases and therefore well-described animal models to understand the influence of NETs in COVID-19 pathogenesis are needed. SARS-CoV-2 infection causes infection and interstitial pneumonia of varying severity in humans and COVID-19 models. Pulmonary as well as peripheral vascular lesions represent a severe, sometimes fatal, disease complication of unknown pathogenesis in COVID-19 patients. Furthermore, neutrophil extracellular traps (NETs), which are known to contribute to vessel inflammation or endothelial damage, have also been shown as potential driver of COVID-19 in humans. Though most studies in animal models describe the pulmonary lesions characterized by interstitial inflammation, type II pneumocyte hyperplasia, edema, fibrin formation and infiltration of macrophages and neutrophils, detailed pathological description of vascular lesions or NETs in COVID-19 animal models are lacking so far. Here we report different types of pulmonary vascular lesions in the golden Syrian hamster model of COVID-19. Vascular lesions included endothelialitis and vasculitis at 3 and 6 days post infection (dpi), and were almost nearly resolved at 14 dpi. Importantly, virus antigen was present in pulmonary lesions, but lacking in vascular alterations. In good correlation to these data, NETs were detected in the lungs of infected animals at 3 and 6 dpi. Hence, the Syrian hamster seems to represent a useful model to further investigate the role of vascular lesions and NETs in COVID-19 pathogenesis.
Keywords: COVID-19; SARS-CoV-2; hamster; neutrophils extracellular traps (NETs); vasculitis.
Copyright © 2021 Becker, Beythien, de Buhr, Stanelle-Bertram, Tuku, Kouassi, Beck, Zickler, Allnoch, Gabriel, von Köckritz-Blickwede and Baumgärtner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

