Consolidation With Pembrolizumab and Nab-Paclitaxel After Induction Platinum-Based Chemotherapy for Advanced Non-Small Cell Lung Cancer
- PMID: 33912470
- PMCID: PMC8074674
- DOI: 10.3389/fonc.2021.666691
Consolidation With Pembrolizumab and Nab-Paclitaxel After Induction Platinum-Based Chemotherapy for Advanced Non-Small Cell Lung Cancer
Abstract
Background: Induction with four cycles of platinum-based chemotherapy was the standard of care for metastatic non-small cell lung cancer (NSCLC) until the approval of immune checkpoint blockade (ICB) in the first-line setting. Switch maintenance therapy has shown promise in improving survival by exposing patients to novel, non-cross-resistant agents earlier in their treatment course.
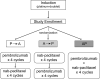
Methods: We performed this open-label, three-arm, randomized phase II study (NCT02684461) to evaluate three sequences of consolidation with pembrolizumab and nab-paclitaxel in patients without progressive disease post induction chemotherapy. Consolidation was either sequential with pembrolizumab for four cycles followed by nab-paclitaxel for four cycles (P→A), nab-paclitaxel followed by pembrolizumab (A→P), or concurrent nab-paclitaxel and pembrolizumab for four cycles (AP).
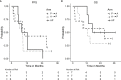
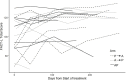
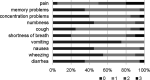
Results: Twenty patients were randomized before the study was closed early due to the approval of first-line checkpoint inhibitors. We found that consolidation is feasible and well tolerated, with 30% of patients experiencing grade 3 toxicity. The median progression-free survival and OS in months (95% CI) in P→A were 10.1 (1.5-NR), 27.6 (1.7-NR); 8.4 (1.2-9.0), 12.7 (4.4-NR) in A→P; and 10.2 (5.1-NR), NR. Quality of life as measured by FACT-L improved in the majority of patients during the course of the study.
Conclusion: Sequential and concurrent consolidation regimens are well tolerated and have encouraging overall survival in patients with metastatic NSCLC.
Keywords: PD-1 antibody; fixed duration; immune checkpoint blockade; sequential therapy; switch maintenance.
Copyright © 2021 Patel, Gerber, Deal, Douglas, Pecot, Lee, Schiller, Dhruva and Weiss.
Conflict of interest statement
SP reports commercial research grants from Shattuck Labs and Dracen Pharmaceuticals. DG reports commercial research grants from AstraZeneca, Karyopharm, and BerGenBio, serving on a steering committee for Bristol-Myers-Squibb and is a paid consultant for Karyopharm, G1 Therapeutics, and Catalyst Therapeutics. CL is a paid consultant for Delcath Systems, Inc. JW reports commercial research grants from Merck and Celgene and has served as a paid consultant for Celgene. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Socinski MA, Schell MJ, Peterman A, Bakri K, Yates S, Gitten R, et al. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J Clin Oncol (2002) 20(5):1335–43. 10.1200/JCO.2002.20.5.1335 - DOI - PubMed
-
- Fidias PM, Dakhil SR, Lyss AP, Loesch DM, Waterhouse DM, Bromund JL, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol (2009) 27(4):591–8. 10.1200/JCO.2008.17.1405 - DOI - PubMed
-
- Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol (2012) 13(3):247–55. 10.1016/S1470-2045(12)70063-3 - DOI - PubMed
-
- Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol (2013) 31(23):2895–902. 10.1200/JCO.2012.47.1102 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

