Effects of prenatal synthetic cannabinoid exposure on the cerebellum of adolescent rat offspring
- PMID: 33912711
- PMCID: PMC8066425
- DOI: 10.1016/j.heliyon.2021.e06730
Effects of prenatal synthetic cannabinoid exposure on the cerebellum of adolescent rat offspring
Abstract
Cannabis is the most commonly used illicit drug worldwide. Recently, cannabis use among young pregnant women has greatly increased. However, prenatal cannabinoid exposure leads to long-lasting cognitive, motor, and behavioral deficits in the offspring and alterations in neural circuitry through various mechanisms. Although these effects have been studied in the hippocampus, the effects of prenatal cannabinoid exposure on the cerebellum are not well elucidated. The cerebellum plays an important role in balance and motor control, as well as cognitive functions such as attention, language, and procedural memories. The aim of this study was to investigate the effects of prenatal cannabinoid exposure on the cerebellum of adolescent offspring. Pregnant rats were treated with synthetic cannabinoid agonist WIN55,212-2, and the offspring were evaluated for various cerebellar markers of oxidative stress, mitochondrial function, and apoptosis. Additionally, signaling proteins associated with glutamate dependent synaptic plasticity were examined. Administration of WIN55,212-2 during pregnancy altered markers of oxidative stress by significantly reducing oxidative stress and nitrite content. Mitochondrial Complex I and Complex IV activities were also enhanced following prenatal cannabinoid exposure. With regard to apoptosis, pP38 levels were significantly increased, and proapoptotic factor caspase-3 activity, pERK, and pJNK levels were significantly decreased. CB1R and GluA1 levels remained unchanged; however, GluN2A was significantly reduced. There was a significant decrease in MAO activity although tyrosine hydroxylase activity was unaltered. Our study indicates that the effects of prenatal cannabinoid exposure on the cerebellum are unique compared to other brain regions by enhancing mitochondrial function and promoting neuronal survival. Further studies are required to evaluate the mechanisms by which prenatal cannabinoid exposure alters cerebellar processes and the impact of these alterations on behavior.
Keywords: Cannabinoid; Cerebellum; Developmental; Mitochondria; Oxidative stress; Prenatal exposure.
© 2021 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
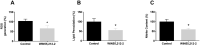





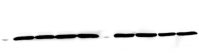









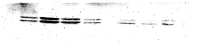

















References
-
- Ahuja M., Buabeid M., Abdel-Rahman E., Majrashi M., Parameshwaran K., Amin R., Ramesh S., Thiruchelvan K., Pondugula S., Suppiramaniam V., Dhanasekaran M. Immunological alteration & toxic molecular inductions leading to cognitive impairment & neurotoxicity in transgenic mouse model of Alzheimer’s disease. Life Sci. 2017;177:49–59. - PubMed
-
- Alhowail A.H., Bloemer J., Majrashi M., Pinky P.D., Bhattacharya S., Yongli Z., Bhattacharya D., Eggert M., Woodie L., Buabeid M.A., Johnson N., Broadwater A., Smith B., Dhanasekaran M., Arnold R.D., Suppiramaniam V. Doxorubicin-induced neurotoxicity is associated with acute alterations in synaptic plasticity, apoptosis, and lipid peroxidation. Toxicol. Mech. Methods. 2019;29:457–466. - PubMed
-
- Amsterdam J. van, Talhout R., Vleeming W., Opperhuizen A. Contribution of monoamine oxidase (MAO) inhibition to tobacco and alcohol addiction. Life Sci. 2006 - PubMed
-
- Bailey J.R., Cunny H.C., Paule M.G., Slikker W. Fetal disposition of delta 9-tetrahydrocannabinol (THC) during late pregnancy in the rhesus monkey. Toxicol. Appl. Pharmacol. 1987;90:315–321. - PubMed
-
- Bénard G., Massa F., Puente N., Lourenço J., Bellocchio L., Soria-Gómez E., Matias I., Delamarre A., Metna-Laurent M., Cannich A., Hebert-Chatelain E., Mulle C., Ortega-Gutiérrez S., Martín-Fontecha M., Klugmann M., Guggenhuber S., Lutz B., Gertsch J., Chaouloff F., López-Rodríguez M.L., Grandes P., Rossignol R., Marsicano G. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat. Neurosci. 2012;15:558–564. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

