Pharmacokinetics of Vancomycin in Pediatric Patients Receiving Intermittent Hemodialysis or Hemodiafiltration
- PMID: 33912750
- PMCID: PMC8071675
- DOI: 10.1016/j.ekir.2021.01.037
Pharmacokinetics of Vancomycin in Pediatric Patients Receiving Intermittent Hemodialysis or Hemodiafiltration
Abstract
Introduction: Vancomycin is a common antibiotic used to treat hemodialysis (HD) or hemodiafiltration (HDF)-related infections in pediatric patients, but optimal dosing remains unknown. This is the first observational study to characterize the pharmacokinetics and evaluate dosing of vancomycin in this population.
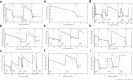
Methods: Eligible patients received IV vancomycin 10 mg/kg per dose postdialysis followed by a series of serum vancomycin concentrations collected before, immediately after, 1 hour after, and 4 hours after dialysis. The pharmacokinetic parameters were estimated using 1- and 2-compartment models and a nonlinear least-squares algorithm.
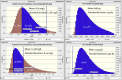
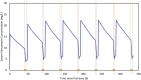
Results: Among 42 vancomycin courses in 16 patients, 1 compartment model had the best fit for observed data. The net drug removal was 43 ± 13% (39% for HD and 50% for HDF) from an average 3-hour HD/HDF session. The mean elimination constant was 0.28 h-1 (standard deviation [SD], 0.11 h-1) during the intradialytic period compared with 0.0049 h-1 (SD, 0.004 h-1) when off dialysis. The mean volume of distribution was 0.65 (SD, 0.19) L/kg. Duration of dialysis session and mode of dialysis (HD vs. HDF) were significant predictors of vancomycin pharmacokinetic parameters. Half-life was shorter for HDF compared with HD (2.1 vs. 3.5 hours).
Conclusions: Based on the simulations, an initial vancomycin dose of 10 mg/kg per dose and redosing postdialysis was optimal to achieve a vancomycin concentration range of 5 to 12 mg/L at 4 hours postdialysis and 24-hour area under the curve over minimum inhibitory concentration of ≥400 hours. Therapeutic drug monitoring is necessary to account for residual variability in vancomycin elimination in pediatric patients receiving HD/HDF.
Keywords: dialysis; drug excretion; pediatrics; pharmacokinetics; vancomycin.
© 2021 International Society of Nephrology. Published by Elsevier Inc.
Figures



References
-
- O'Connor N.R., Corcoran A.M. End-stage renal disease: symptom management and advance care planning. Am Fam Phys. 2012;85:705–710. - PubMed
-
- Daugirdas J.T., Blake P.G., Ing T.S. 5th ed. Wolters Kluwer Health; Amsterdam: 2015. Handbook of Dialysis.
-
- Passlick-Deetjen J., Pohlmeier R. On-line hemodiafiltration. Gold standard or top therapy? Contrib Nephrol. 2002;137:201–211. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

