Ibrutinib Has Time-dependent On- and Off-target Effects on Plasma Biomarkers and Immune Cells in Chronic Lymphocytic Leukemia
- PMID: 33912812
- PMCID: PMC8078281
- DOI: 10.1097/HS9.0000000000000564
Ibrutinib Has Time-dependent On- and Off-target Effects on Plasma Biomarkers and Immune Cells in Chronic Lymphocytic Leukemia
Abstract
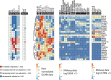
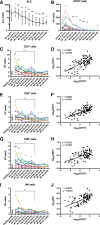
Ibrutinib is a covalently binding inhibitor of the B-cell receptor signaling-mediator Bruton's tyrosine kinase (BTK) with great efficacy in chronic lymphocytic leukemia (CLL). Common side effects like atrial fibrillation (AF), bleeding and infections might be caused by ibrutinib's inhibition of other kinases in non-B cells. Five-year follow-up of plasma biomarkers by proximity extension assay and immune cell numbers by flow cytometry during ibrutinib treatment revealed that 86 of the 265 investigated plasma biomarkers significantly changed during treatment, 74 of which decreased. Among the 12 markers that increased, 6 are associated with cardiovascular diseases and therefore potentially involved in ibrutinib-induced AF. Comparison between healthy donors and X-linked agammaglobulinemia (XLA) patients, who have nonfunctional BTK and essentially lack B cells, showed indicative changes in 53 of the 265 biomarkers while none differed significantly. Hence, neither B cells nor BTK-dependent pathways in other cells seem to influence the levels of the studied plasma biomarkers in healthy donors. Regarding immune cells, the absolute number of T cells, including subsets, decreased, paralleling the decreasing tumor burden. T helper 1 (Th1) cell numbers dropped strongly, while Th2 cells remained relatively stable, causing Th2-skewing. Thus, long-term ibrutinib treatment has a profound impact on the plasma proteome and immune cells in patients with CLL.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Figures






Similar articles
-
Role of Bruton's tyrosine kinase in B cells and malignancies.Mol Cancer. 2018 Feb 19;17(1):57. doi: 10.1186/s12943-018-0779-z. Mol Cancer. 2018. PMID: 29455639 Free PMC article. Review.
-
Current Status of Bruton's Tyrosine Kinase Inhibitor Development and Use in B-Cell Malignancies.Drugs Aging. 2017 Jul;34(7):509-527. doi: 10.1007/s40266-017-0468-4. Drugs Aging. 2017. PMID: 28536906 Review.
-
Noncatalytic Bruton's tyrosine kinase activates PLCγ2 variants mediating ibrutinib resistance in human chronic lymphocytic leukemia cells.J Biol Chem. 2020 Apr 24;295(17):5717-5736. doi: 10.1074/jbc.RA119.011946. Epub 2020 Mar 17. J Biol Chem. 2020. PMID: 32184360 Free PMC article.
-
Ibrutinib treatment improves T cell number and function in CLL patients.J Clin Invest. 2017 Aug 1;127(8):3052-3064. doi: 10.1172/JCI89756. Epub 2017 Jul 17. J Clin Invest. 2017. PMID: 28714866 Free PMC article. Clinical Trial.
-
Leukemia cell proliferation and death in chronic lymphocytic leukemia patients on therapy with the BTK inhibitor ibrutinib.JCI Insight. 2017 Jan 26;2(2):e89904. doi: 10.1172/jci.insight.89904. JCI Insight. 2017. PMID: 28138560 Free PMC article.
Cited by
-
Structure-Function Relationships of Covalent and Non-Covalent BTK Inhibitors.Front Immunol. 2021 Jul 19;12:694853. doi: 10.3389/fimmu.2021.694853. eCollection 2021. Front Immunol. 2021. PMID: 34349760 Free PMC article. Review.
-
Do reduced numbers of plasmacytoid dendritic cells contribute to the aggressive clinical course of COVID-19 in chronic lymphocytic leukaemia?Scand J Immunol. 2022 Apr;95(4):e13153. doi: 10.1111/sji.13153. Epub 2022 Mar 16. Scand J Immunol. 2022. PMID: 35244285 Free PMC article. Review.
-
Effects of ibrutinib on T-cell immunity in patients with chronic lymphocytic leukemia.Front Immunol. 2022 Aug 19;13:962552. doi: 10.3389/fimmu.2022.962552. eCollection 2022. Front Immunol. 2022. PMID: 36059445 Free PMC article. Review.
-
Zanubrutinib: past, present, and future.Blood Cancer J. 2023 Sep 11;13(1):141. doi: 10.1038/s41408-023-00902-x. Blood Cancer J. 2023. PMID: 37696810 Free PMC article. Review.
-
Managing novel therapies and concomitant medications in chronic lymphocytic leukemia: key challenges.Front Pharmacol. 2025 Jan 3;15:1517972. doi: 10.3389/fphar.2024.1517972. eCollection 2024. Front Pharmacol. 2025. PMID: 39830358 Free PMC article. Review.
References
-
- Ortolano S, Hwang IY, Han SB, et al. . Roles for phosphoinositide 3-kinases, Bruton’s tyrosine kinase, and Jun kinases in B lymphocyte chemotaxis and homing. Eur J Immunol. 2006; 36:1285–1295 - PubMed
-
- de Gorter DJ, Beuling EA, Kersseboom R, et al. . Bruton’s tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007; 26:93–104 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
