Differential recruitment of ventral pallidal e-types by behaviorally salient stimuli during Pavlovian conditioning
- PMID: 33912818
- PMCID: PMC8066429
- DOI: 10.1016/j.isci.2021.102377
Differential recruitment of ventral pallidal e-types by behaviorally salient stimuli during Pavlovian conditioning
Abstract
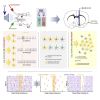
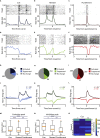
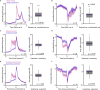
The ventral pallidum (VP) is interfacing striatopallidal and limbic circuits, conveying information about salience and valence crucial to adjusting behavior. However, how VP neuron populations with distinct electrophysiological properties (e-types) represent these variables is not fully understood. Therefore, we trained mice on probabilistic Pavlovian conditioning while recording the activity of VP neurons. Many VP neurons responded to punishment (54%), reward (48%), and outcome-predicting auditory stimuli (32%), increasingly differentiating distinct outcome probabilities through learning. We identified e-types based on the presence of bursts or fast rhythmic discharges and found that non-bursting, non-rhythmic neurons were the most sensitive to reward and punishment. Some neurons exhibited distinct responses of their bursts and single spikes, suggesting a multiplexed coding scheme in the VP. Finally, we demonstrate synchronously firing neuron assemblies, particularly responsive to reinforcing stimuli. These results suggest that electrophysiologically defined e-types of the VP differentially participate in transmitting reinforcement signals during learning.
Keywords: Behavioral Neuroscience; Cellular Neuroscience; Cellular Physiology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures







Similar articles
-
Ventral pallidal representation of pavlovian cues and reward: population and rate codes.J Neurosci. 2004 Feb 4;24(5):1058-69. doi: 10.1523/JNEUROSCI.1437-03.2004. J Neurosci. 2004. PMID: 14762124 Free PMC article.
-
Distinct subpopulations of ventral pallidal cholinergic projection neurons encode valence of olfactory stimuli.Cell Rep. 2024 Apr 23;43(4):114009. doi: 10.1016/j.celrep.2024.114009. Epub 2024 Mar 26. Cell Rep. 2024. PMID: 38536818 Free PMC article.
-
Salience processing by glutamatergic neurons in the ventral pallidum.Sci Bull (Beijing). 2020 Mar 15;65(5):389-401. doi: 10.1016/j.scib.2019.11.029. Epub 2019 Nov 30. Sci Bull (Beijing). 2020. PMID: 36659230
-
Ventral pallidal modulation of aversion processing.Brain Res. 2019 Jun 15;1713:62-69. doi: 10.1016/j.brainres.2018.10.010. Epub 2018 Oct 6. Brain Res. 2019. PMID: 30300634 Review.
-
Opioid modulation of ventral pallidal inputs.Ann N Y Acad Sci. 1999 Jun 29;877:176-201. doi: 10.1111/j.1749-6632.1999.tb09268.x. Ann N Y Acad Sci. 1999. PMID: 10415650 Review.
Cited by
-
Training protocol for probabilistic Pavlovian conditioning in mice using an open-source head-fixed setup.STAR Protoc. 2021 Sep 3;2(3):100795. doi: 10.1016/j.xpro.2021.100795. eCollection 2021 Sep 17. STAR Protoc. 2021. PMID: 34522902 Free PMC article.
-
Basal forebrain innervation of the amygdala: an anatomical and computational exploration.Brain Struct Funct. 2025 Jan 13;230(1):30. doi: 10.1007/s00429-024-02886-1. Brain Struct Funct. 2025. PMID: 39805973 Free PMC article.
-
The antipsychotic drug sulpiride in the ventral pallidum paradoxically impairs learning and induces place preference.Sci Rep. 2022 Nov 10;12(1):19247. doi: 10.1038/s41598-022-23450-z. Sci Rep. 2022. PMID: 36357539 Free PMC article.
-
Parvalbumin-expressing basal forebrain neurons mediate learning from negative experience.Nat Commun. 2024 Jun 7;15(1):4768. doi: 10.1038/s41467-024-48755-7. Nat Commun. 2024. PMID: 38849336 Free PMC article.
-
Cholinergic activity reflects reward expectations and predicts behavioral responses.iScience. 2022 Dec 16;26(1):105814. doi: 10.1016/j.isci.2022.105814. eCollection 2023 Jan 20. iScience. 2022. PMID: 36636356 Free PMC article.
References
-
- Bartho P., Hirase H., Monconduit L., Zugaro M., Harris K.D., Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J. Neurophysiol. 2004;92:600–608. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

