Designing biomaterials for the delivery of RNA therapeutics to stimulate bone healing
- PMID: 33912824
- PMCID: PMC8063862
- DOI: 10.1016/j.mtbio.2021.100105
Designing biomaterials for the delivery of RNA therapeutics to stimulate bone healing
Abstract
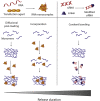
Ribonucleic acids (small interfering RNA, microRNA, and messenger RNA) have been emerging as a promising new class of therapeutics for bone regeneration. So far, however, research has mostly focused on stability and complexation of these oligonucleotides for systemic delivery. By comparison, delivery of RNA nanocomplexes from biomaterial carriers can facilitate a spatiotemporally controlled local delivery of osteogenic oligonucleotides. This review provides an overview of the state-of-the-art in the design of biomaterials which allow for temporal and spatial control over RNA delivery. We correlate this concept of spatiotemporally controlled RNA delivery to the most relevant events that govern bone regeneration to evaluate to which extent tuning of release kinetics is required. In addition, inspired by the physiological principles of bone regeneration, potential new RNA targets are presented. Finally, considerations for clinical translation and upscaled production are summarized to stimulate the design of clinically relevant RNA-releasing biomaterials.
Keywords: Bone regeneration; Controlled release; Oligonucleotide delivery; RNA delivery; mRNA.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

