Testicular germ cell tumors arise in the absence of sex-specific differentiation
- PMID: 33912935
- PMCID: PMC8126408
- DOI: 10.1242/dev.197111
Testicular germ cell tumors arise in the absence of sex-specific differentiation
Abstract
In response to signals from the embryonic testis, the germ cell intrinsic factor NANOS2 coordinates a transcriptional program necessary for the differentiation of pluripotent-like primordial germ cells toward a unipotent spermatogonial stem cell fate. Emerging evidence indicates that genetic risk factors contribute to testicular germ cell tumor initiation by disrupting sex-specific differentiation. Here, using the 129.MOLF-Chr19 mouse model of testicular teratomas and a NANOS2 reporter allele, we report that the developmental phenotypes required for tumorigenesis, including failure to enter mitotic arrest, retention of pluripotency and delayed sex-specific differentiation, were exclusive to a subpopulation of germ cells failing to express NANOS2. Single-cell RNA sequencing revealed that embryonic day 15.5 NANOS2-deficient germ cells and embryonal carcinoma cells developed a transcriptional profile enriched for MYC signaling, NODAL signaling and primed pluripotency. Moreover, lineage-tracing experiments demonstrated that embryonal carcinoma cells arose exclusively from germ cells failing to express NANOS2. Our results indicate that NANOS2 is the nexus through which several genetic risk factors influence tumor susceptibility. We propose that, in the absence of sex specification, signals native to the developing testis drive germ cell transformation.
Keywords: Nanos2; Germ cells; MYC; NODAL; TGCTs; Teratomas.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


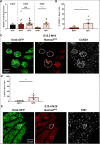
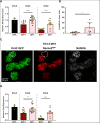
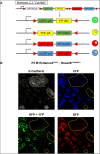


References
-
- Adams, I. and Mclaren, A. (2002). Sexually dimorphic development of mouse primordial germ cells: Switching from oogenesis to spermatogenesis. Development 129, 1155-1164. - PubMed
-
- Anderson, R., Fassler, R., Georges-Labouesse, E., Hynes, R. O., Bader, B. L., Kreidberg, J. A., Schaible, K., Heasman, J. and Wylie, C. (1999). Mouse primordial germ cells lacking beta1 integrins enter the germline but fail to migrate normally to the gonads. Development 126, 1655-1664. - PubMed
-
- Anderson, E. L., Baltus, A. E., Roepers-Gajadien, H. L., Hassold, T. J., de Rooij, D. G., van Pelt, A. M. M. and Page, D. C. (2008). Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl Acad. Sci. USA 105, 14976. 10.1073/pnas.0807297105 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

