LRP10 interacts with SORL1 in the intracellular vesicle trafficking pathway in non-neuronal brain cells and localises to Lewy bodies in Parkinson's disease and dementia with Lewy bodies
- PMID: 33913039
- PMCID: PMC8217053
- DOI: 10.1007/s00401-021-02313-3
LRP10 interacts with SORL1 in the intracellular vesicle trafficking pathway in non-neuronal brain cells and localises to Lewy bodies in Parkinson's disease and dementia with Lewy bodies
Abstract
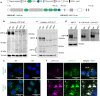
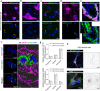
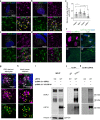
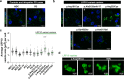
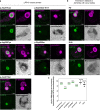
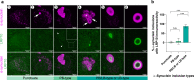
Loss-of-function variants in the low-density lipoprotein receptor-related protein 10 (LRP10) gene have been associated with autosomal-dominant Parkinson's disease (PD), PD dementia, and dementia with Lewy bodies (DLB). Moreover, LRP10 variants have been found in individuals diagnosed with progressive supranuclear palsy and amyotrophic lateral sclerosis. Despite this genetic evidence, little is known about the expression and function of LRP10 protein in the human brain under physiological or pathological conditions. To better understand how LRP10 variants lead to neurodegeneration, we first performed an in-depth characterisation of LRP10 expression in post-mortem brains and human-induced pluripotent stem cell (iPSC)-derived astrocytes and neurons from control subjects. In adult human brain, LRP10 is mainly expressed in astrocytes and neurovasculature but undetectable in neurons. Similarly, LRP10 is highly expressed in iPSC-derived astrocytes but cannot be observed in iPSC-derived neurons. In astrocytes, LRP10 is present at trans-Golgi network, plasma membrane, retromer, and early endosomes. Interestingly, LRP10 also partially co-localises and interacts with sortilin-related receptor 1 (SORL1). Furthermore, although LRP10 expression and localisation in the substantia nigra of most idiopathic PD and DLB patients and LRP10 variant carriers diagnosed with PD or DLB appeared unchanged compared to control subjects, significantly enlarged LRP10-positive vesicles were detected in a patient carrying the LRP10 p.Arg235Cys variant. Last, LRP10 was detected in Lewy bodies (LB) at late maturation stages in brains from idiopathic PD and DLB patients and in LRP10 variant carriers. In conclusion, high LRP10 expression in non-neuronal cells and undetectable levels in neurons of control subjects indicate that LRP10-mediated pathogenicity is initiated via cell non-autonomous mechanisms, potentially involving the interaction of LRP10 with SORL1 in vesicle trafficking pathways. Together with the specific pattern of LRP10 incorporation into mature LBs, these data support an important mechanistic role for disturbed vesicle trafficking and loss of LRP10 function in neurodegenerative diseases.
Keywords: Astrocytes; Dementia with Lewy bodies (DLB); LRP10; Lewy bodies; Parkinson’s disease (PD); Vesicle trafficking.
Conflict of interest statement
VB receives honoraria from the International Parkinson and Movement Disorder Society for serving as Chair of the MDS International Congress Program Committee 2019-2021; from Elsevier Ltd, for serving as co-Editor-in-Chief of Parkinsonism & Related Disorders; and from Springer, for serving as Section Editor of Current Neurology and Neuroscience Reports. WDJvdB was financially supported by grants from Amsterdam Neuroscience, Dutch Research council (ZonMW 70-73305-98-106; 70-73305-98-102; 40-46000-98-101), Stichting Parkinson Fonds (Insula 2014-2019), Alzheimer association (AARF-18-566459), MJ Fox foundation (17253) and Parkinson Association (2020-G01). WDJvdB performed contract research for Hoffmann-La Roche, Roche Tissue Diagnostics, Crossbeta Sciences, Lundbeck and received research consumables from Hoffmann-La Roche and Prothena. All other authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

