Is group testing ready for prime-time in disease identification?
- PMID: 33913183
- PMCID: PMC8742170
- DOI: 10.1002/sim.9003
Is group testing ready for prime-time in disease identification?
Abstract
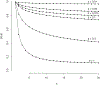
Large-scale disease screening is a complicated process in which high costs must be balanced against pressing public health needs. When the goal is screening for infectious disease, one approach is group testing in which samples are initially tested in pools and individual samples are retested only if the initial pooled test was positive. Intuitively, if the prevalence of infection is small, this could result in a large reduction of the total number of tests required. Despite this, the use of group testing in medical studies has been limited, largely due to skepticism about the impact of pooling on the accuracy of a given assay. While there is a large body of research addressing the issue of testing errors in group testing studies, it is customary to assume that the misclassification parameters are known from an external population and/or that the values do not change with the group size. Both of these assumptions are highly questionable for many medical practitioners considering group testing in their study design. In this article, we explore how the failure of these assumptions might impact the efficacy of a group testing design and, consequently, whether group testing is currently feasible for medical screening. Specifically, we look at how incorrect assumptions about the sensitivity function at the design stage can lead to poor estimation of a procedure's overall sensitivity and expected number of tests. Furthermore, if a validation study is used to estimate the pooled misclassification parameters of a given assay, we show that the sample sizes required are so large as to be prohibitive in all but the largest screening programs.
Keywords: disease screening; epidemiology; group testing; measurement error.
© 2021 John Wiley & Sons, Ltd.
Figures
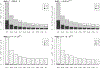
Comment in
-
Comment on "Is Group Testing Ready for Prime Time in Disease Identification?".Stat Med. 2021 Jul 30;40(17):3889-3891. doi: 10.1002/sim.9078. Stat Med. 2021. PMID: 34251035 No abstract available.
-
Rejoinder to discussion on Is group testing ready for prime-time in disease identification?Stat Med. 2021 Jul 30;40(17):3892-3894. doi: 10.1002/sim.9033. Stat Med. 2021. PMID: 34251036 Free PMC article. No abstract available.
-
Discussion of "Is group testing ready for prime-time in disease Identification?" by Haber, Malinovsky, and Albert, Statistics in Medicine, 2021.Stat Med. 2021 Jul 30;40(17):3887-3888. doi: 10.1002/sim.8989. Stat Med. 2021. PMID: 34251037 Free PMC article. No abstract available.
-
Discussion on "Is group testing ready for prime-time in disease identification".Stat Med. 2021 Jul 30;40(17):3881-3886. doi: 10.1002/sim.8988. Stat Med. 2021. PMID: 34251038 Free PMC article. No abstract available.
References
-
- Dorfman R. The detection of defective members of large populations. Ann Math Stat. 1943;14(4):436–440.
-
- Aldridge M, Johnson O, Scarlett J. Group testing: an information theory perspective. Found Trends Commun Inf Theory. 2019;15(3–4):196–392.
-
- Bar-Lev SK, Boxma O, Stadje W, Van der Duyn Schouten FA. A two-stage group testing model for infections with window periods. Methodol Comput Appl Probab. 2010;12(3):309–322.
-
- Zhigljavsky A. Probabilistic existence theorems in group testing. J Stat Plann Infer. 2003;115(1):1–43.
-
- Macula AJ. Probabilistic nonadaptive group testing in the presence of errors and DNA library screening. Ann Comb. 1999;3(1):61–69.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
