Get closer and make hotspots: liquid-liquid phase separation in plants
- PMID: 33913240
- PMCID: PMC8097390
- DOI: 10.15252/embr.202051656
Get closer and make hotspots: liquid-liquid phase separation in plants
Abstract
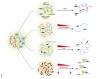
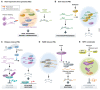
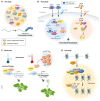
Liquid-liquid phase separation (LLPS) facilitates the formation of membraneless compartments in a cell and allows the spatiotemporal organization of biochemical reactions by concentrating macromolecules locally. In plants, LLPS defines cellular reaction hotspots, and stimulus-responsive LLPS is tightly linked to a variety of cellular and biological functions triggered by exposure to various internal and external stimuli, such as stress responses, hormone signaling, and temperature sensing. Here, we provide an overview of the current understanding of physicochemical forces and molecular factors that drive LLPS in plant cells. We illustrate how the biochemical features of cellular condensates contribute to their biological functions. Additionally, we highlight major challenges for the comprehensive understanding of biological LLPS, especially in view of the dynamic and robust organization of biochemical reactions underlying plastic responses to environmental fluctuations in plants.
Keywords: condensate; intrinsically disordered protein; liquid-liquid phase separation; multivalent interaction; prion-like domain.
© 2021 The Authors.
Conflict of interest statement
The authors declared that they have no conflict of interest.
Figures

References
-
- Alba MM, Pages M (1998) Plant proteins containing RNA‐recognition motif. Trends Plant Sci 3: 15–21
-
- Alberti S (2017) Phase separation in biology. Curr Biol 27: R1097–R1102 - PubMed
-
- Ang LH, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, Deng XW (1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell 1: 213–222 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

