Automated Radiosynthesis of cis- and trans-4-[18F]Fluoro-l-proline Using [18F]Fluoride
- PMID: 33913318
- PMCID: PMC8524414
- DOI: 10.1021/acs.joc.1c00755
Automated Radiosynthesis of cis- and trans-4-[18F]Fluoro-l-proline Using [18F]Fluoride
Abstract
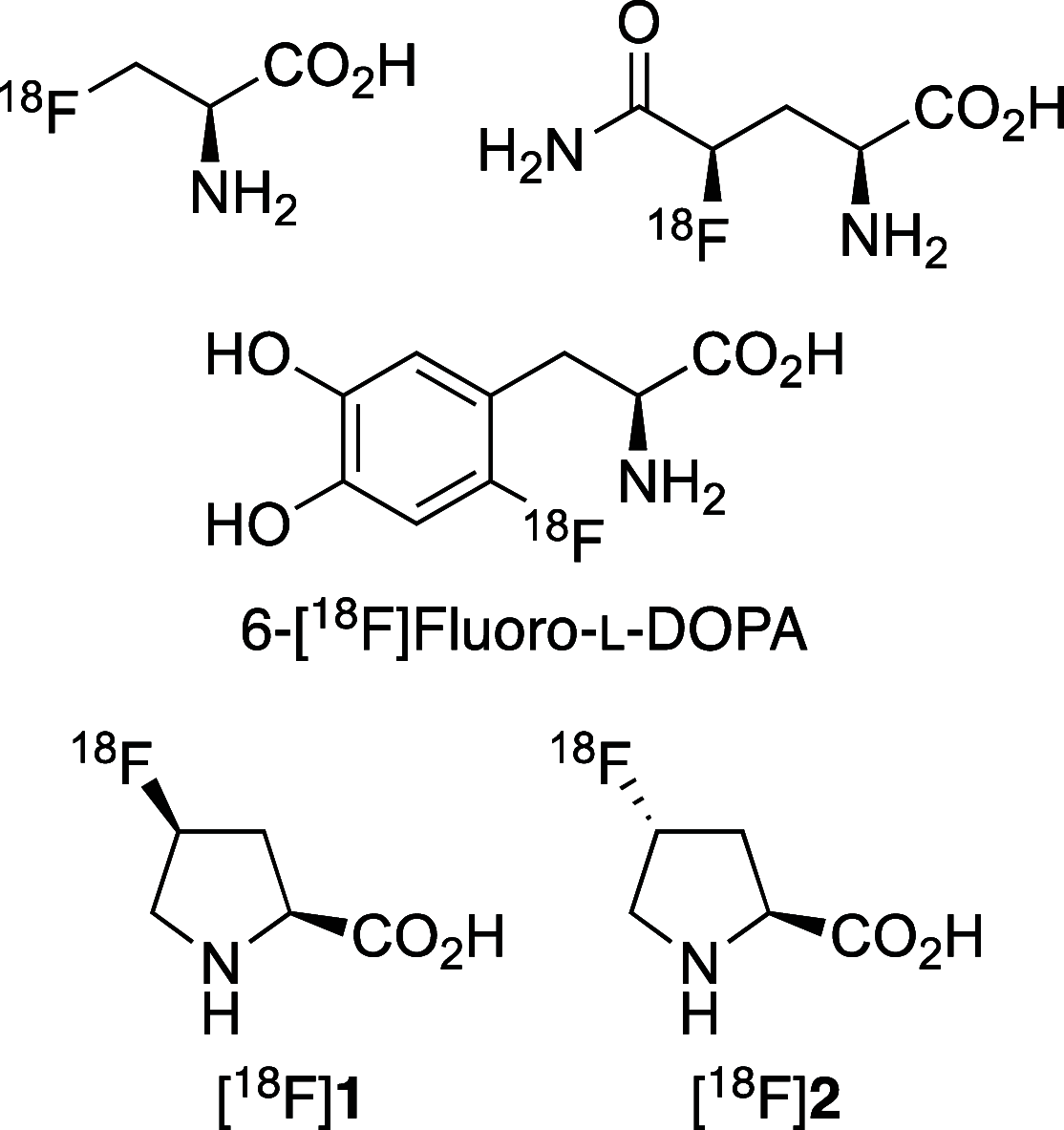
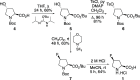
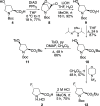
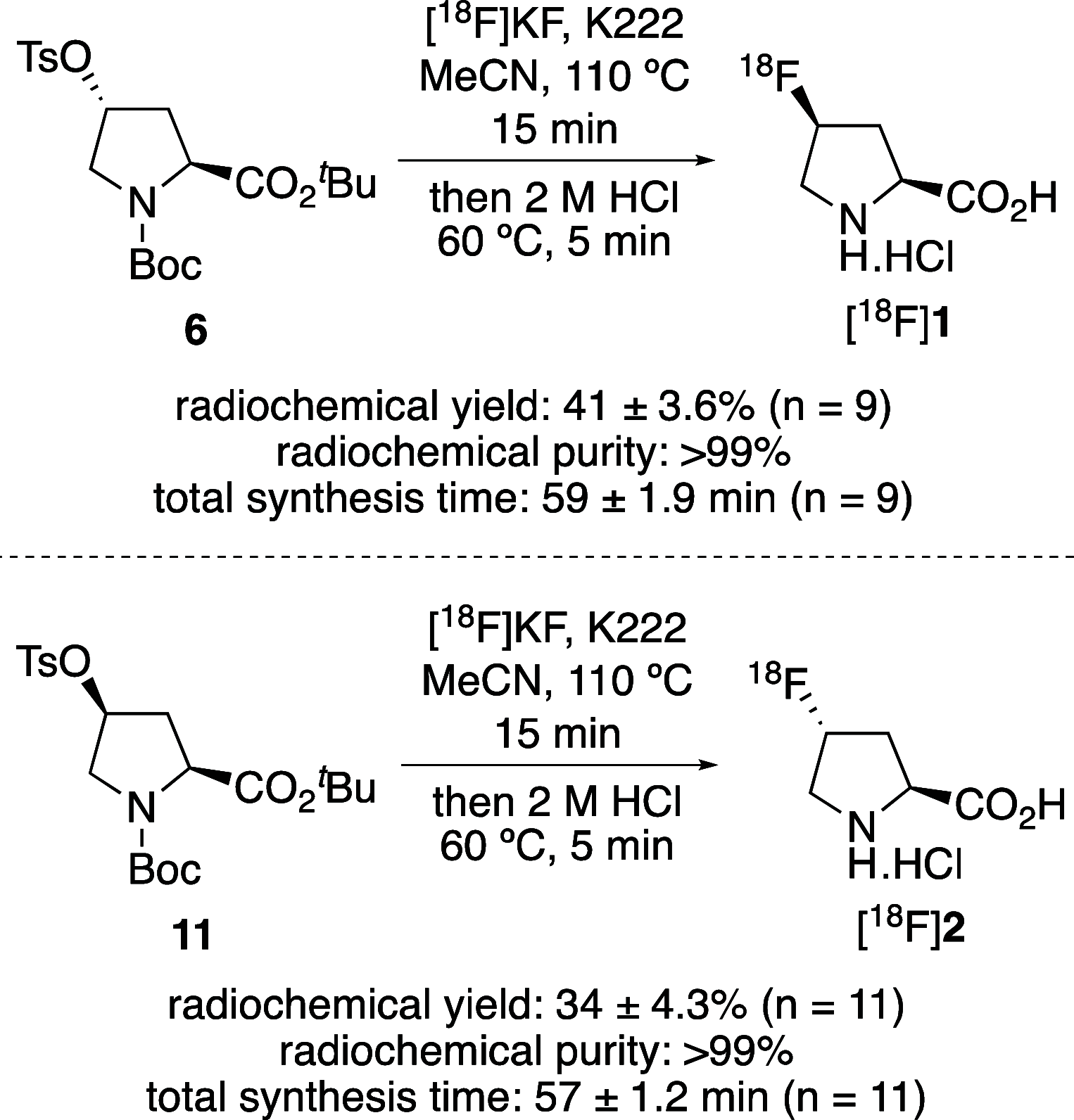
The positron emission tomography imaging agents cis- and trans-4-[18F]fluoro-l-proline are used for the detection of numerous diseases such as pulmonary fibrosis and various carcinomas. These imaging agents are typically prepared by nucleophilic fluorination of 4-hydroxy-l-proline derivatives, with [18F]fluoride, followed by deprotection. Although effective radiofluorination reactions have been developed, the overall radiosynthesis process is suboptimal due to deprotection methods that are performed manually, require multiple steps, or involve harsh conditions. Here we describe the development of two synthetic routes that allow access to precursors, which undergo highly selective radiofluorination reactions and rapid deprotection, under mild acidic conditions. These methods were found to be compatible with automation, avoiding manual handling of radioactive intermediates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

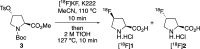
References
-
-
For reviews, see:
- Wagner I.; Musso H. New Naturally Occurring Amino Acids. Angew. Chem., Int. Ed. Engl. 1983, 22, 816–828. 10.1002/anie.198308161. - DOI
- Greenstein J. P.; Winitz M.. Chemistry of the Amino Acids; Krieger, 1984, Vols. 1–3.
- Barrett G. E., Ed. Chemistry and Biochemistry of the Amino Acids; Chapman and Hall: London, 1985.
- Breslow R.; Chmielewski J.; Foley D.; Johnson B.; Kumabe N.; Varney M.; Mehra R. Optically Active Amino Acid Synthesis by Artificial Transaminase Enzymes. Tetrahedron 1988, 44, 5515–5524. 10.1016/S0040-4020(01)86057-9. - DOI
-
-
- Dougherty D. A. Unnatural Amino Acids as Probes of Protein Structure and Function. Curr. Opin. Chem. Biol. 2000, 4, 645–652. 10.1016/S1367-5931(00)00148-4. - DOI - PubMed
- Walsh C. T.; O’Brien R. V.; Khosla C. Nonproteinogenic Amino Acid Building Blocks for Nonribosomal Peptide and Hybrid Polyketide Scaffolds. Angew. Chem., Int. Ed. 2013, 52, 7098–7124. 10.1002/anie.201208344. - DOI - PMC - PubMed
- Lang K.; Chin J. W. Cellular Incorporation of Unnatural Amino Acids and Bioorthogonal Labeling of Proteins. Chem. Rev. 2014, 114, 4764–4806. 10.1021/cr400355w. - DOI - PubMed
-
- Krueger A. T.; Imperiali B. Fluorescent Amino Acids: Modular Building Blocks for the Assembly of New Tools for Chemical Biology. ChemBioChem 2013, 14, 788–799. 10.1002/cbic.201300079. - DOI - PubMed
- Kubota R.; Hamachi I. Protein Recognition using Synthetic Small-Molecular Binders Toward Optical Protein Sensing In Vitro and in Live Cells. Chem. Soc. Rev. 2015, 44, 4454–4471. 10.1039/C4CS00381K. - DOI - PubMed
- Harkiss A. H.; Sutherland A. Recent Advances in the Synthesis and Application of Fluorescent α-Amino Acids. Org. Biomol. Chem. 2016, 14, 8911–8921. 10.1039/C6OB01715K. - DOI - PubMed
- Cheng Z.; Kuru E.; Sachdeva A.; Vendrell M. Fluorescent Amino Acids as Versatile Building Block for Chemical Biology. Nat. Rev. Chem. 2020, 4, 275–290. 10.1038/s41570-020-0186-z. - DOI - PubMed
-
- Jager P. L.; Vaalburg W.; Pruim J.; de Vries E. G. E.; Langen K.-J.; Piers D. A. Radiolabeled Amino Acids: Basic Aspects and Clinical Applications in Oncology. J. Nucl. Med. 2001, 42, 432–445. - PubMed
- McConathy J.; Yu W.; Jarkas N.; Seo W.; Schuster D. M.; Goodman M. M. Radiohalogenated Nonnatural Amino Acids as PET and SPECT Tumour Imaging Agents. Med. Res. Rev. 2012, 32, 868–905. 10.1002/med.20250. - DOI - PubMed
- Ermert J.; Coenen H. H. Methods for 11C and 18F-Labelling of Amino Acids and Derivatives for Positron Emission Tomography Imaging. J. Labelled Compd. Radiopharm. 2013, 56, 225–236. 10.1002/jlcr.2996. - DOI - PubMed
- Huang C.; McConathy J. Radiolabeled Amino Acids for Oncologic Imaging. J. Nucl. Med. 2013, 54, 1007–1010. 10.2967/jnumed.112.113100. - DOI - PubMed
- Sun A.; Liu X.; Tang G. Carbon-11 and Fluorine-18 Labeled Amino Acid Tracers for Positron Emission Tomography Imaging of Tumours. Front. Chem. 2018, 5, 124.10.3389/fchem.2017.00124. - DOI - PMC - PubMed
-
- Darcourt J.; Schiazza A.; Sapin N.; Dufour M.; Ouvrier M. J.; Benisvy D.; Fontana X.; Koulibaly P. M. 18F-FDOPA PET for the Diagnosis of Parkinson Syndromes. Q. J. Nucl. Med. Mol. Imaging 2014, 58, 355–365. - PubMed
- Mossine A. V.; Tanzey S. S.; Brooks A. F.; Makaravage K. J.; Ichiishi N.; Miller J. M.; Henderson B. D.; Skaddan M. B.; Sanford M. S.; Scott P. J. H. One-pot Synthesis of High Molar Activity 6-[18F]Fluoro-L-DOPA by Cu-mediated Fluorination of a BPin Precursor. Org. Biomol. Chem. 2019, 17, 8701–8705. 10.1039/C9OB01758E. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

