PRSS50 is a testis protease responsible for proper sperm tail formation and function
- PMID: 33913480
- PMCID: PMC8077533
- DOI: 10.1242/dev.197558
PRSS50 is a testis protease responsible for proper sperm tail formation and function
Abstract
Multiple morphological abnormalities of the sperm flagella (MMAF) are a major cause of asthenoteratozoospermia. We have identified protease serine 50 (PRSS50) as having a crucial role in sperm development, because Prss50-null mice presented with impaired fertility and sperm tail abnormalities. PRSS50 could also be involved in centrosome function because these mice showed a threefold increase in acephalic sperm (head-tail junction defect), sperm with multiple heads (spermatid division defect) and sperm with multiple tails, including novel two conjoined sperm (complete or partial parts of several flagellum on the same plasma membrane). Our data support that, in the testis, as in tumorigenesis, PRSS50 activates NFκB target genes, such as the centromere protein leucine-rich repeats and WD repeat domain-containing protein 1 (LRWD1), which is required for heterochromatin maintenance. Prss50-null testes have increased IκκB, and reduced LRWD1 and histone expression. Low levels of de-repressed histone markers, such as H3K9me3, in the Prss50-null mouse testis may cause increases in post-meiosis proteins, such as AKAP4, affecting sperm formation. We provide important insights into the complex mechanisms of sperm development, the importance of testis proteases in fertility and a novel mechanism for MMAF.
Keywords: AKAP4; Centrosome; H3K9me3; Infertility; IκB; LRWD1; MMAF; Midpiece; Mitochondria; NFκB; PRSS50; SEPT12; Sperm tail.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
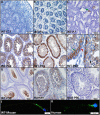

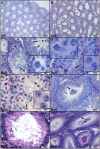

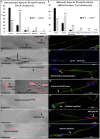
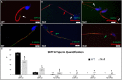
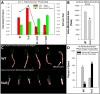

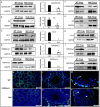
Similar articles
-
Bi-allelic Loss-of-function Variants in CFAP58 Cause Flagellar Axoneme and Mitochondrial Sheath Defects and Asthenoteratozoospermia in Humans and Mice.Am J Hum Genet. 2020 Sep 3;107(3):514-526. doi: 10.1016/j.ajhg.2020.07.010. Epub 2020 Aug 12. Am J Hum Genet. 2020. PMID: 32791035 Free PMC article.
-
CCDC181 is required for sperm flagellum biogenesis and male fertility in mice.Zool Res. 2024 Sep 18;45(5):1061-1072. doi: 10.24272/j.issn.2095-8137.2024.075. Zool Res. 2024. PMID: 39245650 Free PMC article.
-
Insight on multiple morphological abnormalities of sperm flagella in male infertility: what is new?Asian J Androl. 2020 May-Jun;22(3):236-245. doi: 10.4103/aja.aja_53_19. Asian J Androl. 2020. PMID: 31210147 Free PMC article. Review.
-
Leucine-rich repeat and WD repeat-containing protein 1 is recruited to pericentric heterochromatin by trimethylated lysine 9 of histone H3 and maintains heterochromatin silencing.J Biol Chem. 2012 Apr 27;287(18):15024-33. doi: 10.1074/jbc.M111.337980. Epub 2012 Mar 15. J Biol Chem. 2012. PMID: 22427655 Free PMC article.
-
The genetic architecture of morphological abnormalities of the sperm tail.Hum Genet. 2021 Jan;140(1):21-42. doi: 10.1007/s00439-020-02113-x. Epub 2020 Jan 16. Hum Genet. 2021. PMID: 31950240 Review.
Cited by
-
The Molecular Basis of Multiple Morphological Abnormalities of Sperm Flagella and Its Impact on Clinical Practice.Genes (Basel). 2024 Oct 13;15(10):1315. doi: 10.3390/genes15101315. Genes (Basel). 2024. PMID: 39457439 Free PMC article. Review.
-
Proteasome-Associated Proteins, PA200 and ECPAS, Are Essential for Murine Spermatogenesis.Biomolecules. 2023 Mar 24;13(4):586. doi: 10.3390/biom13040586. Biomolecules. 2023. PMID: 37189334 Free PMC article.
-
Divergence of X-linked trans regulatory proteins and the misexpression of gene targets in sterile Drosophila pseudoobscura hybrids.BMC Genomics. 2022 Jan 6;23(1):30. doi: 10.1186/s12864-021-08267-w. BMC Genomics. 2022. PMID: 34991488 Free PMC article.
-
Knockout of ovary serine protease Leads to Ovary Deformation and Female Sterility in the Asian Corn Borer, Ostrinia furnacalis.Int J Mol Sci. 2023 Nov 14;24(22):16311. doi: 10.3390/ijms242216311. Int J Mol Sci. 2023. PMID: 38003502 Free PMC article.
-
PRSS50-mediated inhibition of MKP3/ERK signaling is crucial for meiotic progression and sperm quality.Zool Res. 2024 Sep 18;45(5):1037-1047. doi: 10.24272/j.issn.2095-8137.2023.388. Zool Res. 2024. PMID: 39147718 Free PMC article.
References
-
- Adams, S. R., Maezawa, S., Alavattam, K. G., Abe, H., Sakashita, A., Shroder, M., Broering, T. J., Sroga Rios, J., Thomas, M. A., Lin, X.et al. (2018). RNF8 and SCML2 cooperate to regulate ubiquitination and H3K27 acetylation for escape gene activation on the sex chromosomes. PLoS Genet. 14, e1007233. 10.1371/journal.pgen.1007233 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

