Efficacy of secukinumab and adalimumab in patients with psoriatic arthritis and concomitant moderate-to-severe plaque psoriasis: results from EXCEED, a randomized, double-blind head-to-head monotherapy study
- PMID: 33913511
- PMCID: PMC9291158
- DOI: 10.1111/bjd.20413
Efficacy of secukinumab and adalimumab in patients with psoriatic arthritis and concomitant moderate-to-severe plaque psoriasis: results from EXCEED, a randomized, double-blind head-to-head monotherapy study
Abstract
Background: Secukinumab [an interleukin (IL)-17A inhibitor] has demonstrated significantly higher efficacy vs. etanercept (a tumour necrosis factor inhibitor) and ustekinumab (an IL-12/23 inhibitor) in patients with moderate-to-severe plaque psoriasis.
Objectives: To report 52-week results from a prespecified analysis of patients with active psoriatic arthritis (PsA) having concomitant moderate-to-severe plaque psoriasis from the head-to-head EXCEED monotherapy study comparing secukinumab with adalimumab.
Methods: Patients were randomized to receive secukinumab 300 mg via subcutaneous injection at baseline, week 1-4, and then every 4 weeks until week 48 or adalimumab 40 mg via subcutaneous injection every 2 weeks from baseline until week 50. Assessments in patients with concomitant moderate-to-severe psoriasis, defined as having affected body surface area > 10% or Psoriasis Area and Severity Index (PASI) ≥ 10 at baseline, included musculoskeletal, skin and quality-of-life outcomes. Missing data were handled using multiple imputation.
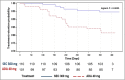
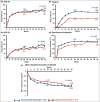
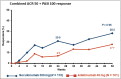
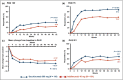
Results: Of the 853 patients [secukinumab (N = 426), adalimumab (N = 427)], 211 (24·7%) had concomitant moderate-to-severe psoriasis [secukinumab (N = 110, 25·8%), adalimumab (N = 101, 23·7%)]. Up to week 50, 5·5% of patients discontinued secukinumab vs.17·8% in the adalimumab group. The proportion of patients who achieved American College of Rheumatology (ACR) 20 response was 76·4% with secukinumab vs. 68·3% with adalimumab (P = 0·175), PASI 100 response was 39·1% vs. 23·8% (P = 0·013), and simultaneous improvement in ACR 50 and PASI 100 response at week 52 was 28·2% vs. 17·7%, respectively (P = 0·06). Secukinumab demonstrated consistently higher responses vs. adalimumab across skin endpoints.
Conclusions: This prespecified analysis in PsA patients with concomitant moderate-to-severe plaque psoriasis in the EXCEED study provides further evidence that IL-17 inhibitors offer a comprehensive biological treatment to manage the concomitant features of psoriasis and PsA.
© 2021 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Conflict of interest statement
A.B.G. has received honoraria for acting as an advisory board member and for consulting from Anaptyps Bio, Avotres Therapeutics, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb Co., Incyte, Dermavant, Janssen Inc. LEO Pharma, Eli Lilly, Novartis, Sun Pharmaceutical Industries, UCB and Xbiotech (stock options). A.B.G. has received research/educational grants from Boehringer Ingelheim, Incyte, Janssen Inc., Novartis, UCB, Xbiotech and Sun Pharma (all educational and research grant income went to the Icahn School of Medicine at Mount Sinai). J.F.M. has acted as a consultant for Merck, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB Pharma, Celgene, Sanofi, Regeneron, Arena, Sun Pharma, Biogen, Pfizer, EMD Serono, Avotres and LEO Pharma. K.R. has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Centocor, Covagen, Dermira, Forward Pharma, Fresenius Medical Care, Galapagos, GlaxoSmithKline, Janssen‐Cilag, Kyowa Kirin, LEO, Lilly, Medac, Merck Sharp & Dohme, Novartis, Miltenyi Biotec, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant and Xenoport. F.B. has received research grants from Pfizer, Janssen, Chugai, Celgene and Roche. F.B. has also received consultancy/speaker fees from Pfizer, AbbVie, Amgen, Sanofi, Lilly, Novartis, Gilead, Genzyme, Boehringer, Janssen, MSD, Celgene, Roche and Chugai, BMS, UCB Pharma. P.N. has received grant/research support from AbbVie, BMS, Celgene, Eli Lilly, Gilead Sciences, Inc, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Amgen, Roche and Sanofi‐Aventis. P.N. has been a consultant for AbbVie, BMS, Celgene, Eli Lilly, Gilead Sciences, Inc., Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Amgen, Roche and Sanofi‐Aventis. P.N. has also participated in a speakers bureau for AbbVie, BMS, Celgene, Eli Lilly, Gilead Sciences, Inc, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Amgen, Roche and Sanofi‐Aventis. C.E.M.G. has received honoraria and/or research support from AbbVie, Amgen, Almirall, BMS, Boehringer Ingelheim Celgene, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sun Pharma and UCB Pharma. W.B., P.P. and L.P. are shareholders of and employees of Novartis. I.B.M. has received grant/research support from AbbVie, Janssen, Novartis, Lilly, Celgene, UCB Pharma, BMS, Boehringer Ingelheim, AstraZeneca, Pfizer and has acted as a consultant for AbbVie, Janssen, Novartis, Lilly, Celgene, Compugen, UCB Pharma, BMS, Boehringer Ingelheim, AstraZeneca and Pfizer.
Figures

Comment in
-
Head-to-head trials in psoriasis and psoriatic arthritis: how to conclude?Br J Dermatol. 2021 Dec;185(6):1085. doi: 10.1111/bjd.20771. Epub 2021 Oct 20. Br J Dermatol. 2021. PMID: 34668197 No abstract available.
References
-
- Ciocon DH, Kimball AB. Psoriasis and psoriatic arthritis: separate or one and the same? Br J Dermatol 2007; 157:850–60. - PubMed
-
- Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population‐based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey. Am J Clin Dermatol 2016; 17:87–97. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

