The causal role of auditory cortex in auditory working memory
- PMID: 33913809
- PMCID: PMC8169109
- DOI: 10.7554/eLife.64457
The causal role of auditory cortex in auditory working memory
Abstract
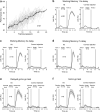
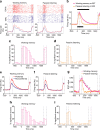
Working memory (WM), the ability to actively hold information in memory over a delay period of seconds, is a fundamental constituent of cognition. Delay-period activity in sensory cortices has been observed in WM tasks, but whether and when the activity plays a functional role for memory maintenance remains unclear. Here, we investigated the causal role of auditory cortex (AC) for memory maintenance in mice performing an auditory WM task. Electrophysiological recordings revealed that AC neurons were active not only during the presentation of the auditory stimulus but also early in the delay period. Furthermore, optogenetic suppression of neural activity in AC during the stimulus epoch and early delay period impaired WM performance, whereas suppression later in the delay period did not. Thus, AC is essential for information encoding and maintenance in auditory WM task, especially during the early delay period.
Keywords: auditory cortex; mice; mouse; neuroscience; working memory.
Plain language summary
Working memory is the ability to hold information in your head for a few seconds while making decisions, planning or applying logical reasoning to problem solving. It is a fundamental component of cognition, and yet it remains unclear where working memory is stored in the brain. The prefrontal cortex – the front lobe of the brain – is likely the main hub of working memory, since it is responsible for executive functions, such as decision making and planning. This idea is supported by experiments showing sustained brain activity in the prefrontal cortex during working memory tasks. Lesions in that part of the brain also lead to profound deficits in working memory. However, there is increasing evidence that other parts of the brain which process sensory information also participate in retaining working memory. The auditory cortex, which processes sound, is one such candidate. To find out whether the auditory cortex has a role to play in working memory, Yu, Hu, Shi et al. trained mice to lick a water spout after hearing the same sound twice in a row, 1.5 seconds apart, and then measured the activities of the mice’s neurons. This showed that neurons in the auditory cortex were active not only when the mice were presented with sound cues, but also for a short time during the delay period between sounds. Yu, Hu, Shi et al. then manipulated this neurons to inactivate them for a fraction of a second after the first sound, which resulted in the animals’ working memory was impaired. However, suppressing the activity of the auditory cortex cells in the later stages of the sound delay period had no effect on working memory. These results indicate that although the auditory cortex may not be involved in storing information for the entire working memory process, it is crucial for encoding of auditory information. In summary, this work uncovers how neurons in the auditory cortex underlie working memory. Further research focusing on these neurons could explain how working memory deteriorates with age, or why it is impaired in people with learning difficulties.
© 2021, Yu et al.
Conflict of interest statement
LY, JH, CS, LZ, MT, JZ, JX No competing interests declared
Figures








References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

