Image-guided percutaneous ablation for the treatment of lung malignancies: current state of the art
- PMID: 33914187
- PMCID: PMC8085189
- DOI: 10.1186/s13244-021-00997-5
Image-guided percutaneous ablation for the treatment of lung malignancies: current state of the art
Abstract
Image-guided percutaneous lung ablation has proven to be a valid treatment alternative in patients with early-stage non-small cell lung carcinoma or oligometastatic lung disease. Available ablative modalities include radiofrequency ablation, microwave ablation, and cryoablation. Currently, there are no sufficiently representative studies to determine significant differences between the results of these techniques. However, a common feature among them is their excellent tolerance with very few complications. For optimal treatment, radiologists must carefully select the patients to be treated, perform a refined ablative technique, and have a detailed knowledge of the radiological features following lung ablation. Although no randomized studies comparing image-guided percutaneous lung ablation with surgery or stereotactic radiation therapy are available, the current literature demonstrates equivalent survival rates. This review will discuss image-guided percutaneous lung ablation features, including available modalities, approved indications, possible complications, published results, and future applications.
Keywords: Metastatic lung disease; Non-small cell lung carcinoma; Percutaneous thermal ablation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


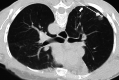

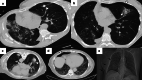
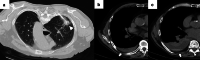
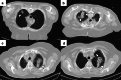


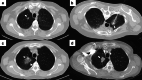
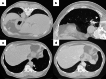
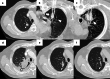
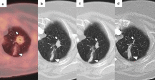
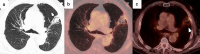


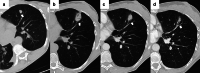

References
-
- Detterbeck FC, Chansky K, Groome P, et al. The IASLC lung cancer staging project: methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the forthcoming (eighth) edition of the TNM classification of lung cancer. J Thorac Oncol. 2016;11:1433–1446. doi: 10.1016/j.jtho.2016.06.028. - DOI - PubMed
-
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small-cell lung cancer. J Clin Oncol. 2014;32:2456–2462. doi: 10.1200/JCO.2013.53.4115. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

