Maturation conditions, post-ovulatory age, medium pH, and ER stress affect [Ca2+]i oscillation patterns in mouse oocytes
- PMID: 33914207
- PMCID: PMC8266968
- DOI: 10.1007/s10815-021-02100-9
Maturation conditions, post-ovulatory age, medium pH, and ER stress affect [Ca2+]i oscillation patterns in mouse oocytes
Abstract
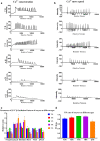
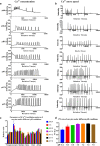
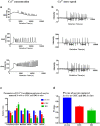
Insufficiency of oocyte activation impairs the subsequent embryo development in assisted reproductive technology (ART). Intracellular Ca2+ concentration ([Ca2+]i) oscillations switch the oocytes to resume the second meiosis and initiate embryonic development. However, the [Ca2+]i oscillation patterns in oocytes are poorly characterized. In this study, we investigated the effects of various factors, such as the oocytes age, pH, cumulus cells, in vitro or in vivo maturation, and ER stress on [Ca2+]i oscillation patterns and pronuclear formation after parthenogenetic activation of mouse oocytes. Our results showed that the oocytes released to the oviduct at 17 h post-human chorionic gonadotrophin (hCG) displayed a significantly stronger [Ca2+]i oscillation, including higher frequency, shorter cycle, and higher peak, compared with oocytes collected at earlier or later time points. [Ca2+]i oscillations in acidic conditions (pH 6.4 and 6.6) were significantly weaker than those in neutral and mildly alkaline conditions (pH from 6.8 to 7.6). In vitro-matured oocytes showed reduced frequency and peak of [Ca2+]i oscillations compared with those matured in vivo. In vitro-matured oocytes from the cumulus-oocyte complexes (COCs) showed a significantly higher frequency, shorter cycle, and higher peak compared with the denuded oocytes (DOs). Finally, endoplasmic reticulum stress (ER stress) severely affected the parameters of [Ca2+]i oscillations, including elongated cycles and lower frequency. The pronuclear (PN) rate of oocytes after parthenogenetic activation was correlated with [Ca2+]i oscillation pattern, decreasing with oocyte aging, cumulus removal, acidic pH, and increasing ER stress. These results provide fundamental but critical information for the mechanism of how these factors affect oocyte activation.
Keywords: Calcium oscillations; Endoplasmic reticulum stress; In vitro oocyte maturation techniques; Oocytes; Parthenogenesis.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Mechanistic insights into the reduced developmental capacity of in vitro matured oocytes and importance of cumulus cells in oocyte quality determination.J Cell Physiol. 2020 Dec;235(12):9743-9751. doi: 10.1002/jcp.29786. Epub 2020 May 16. J Cell Physiol. 2020. PMID: 32415704
-
Oocyte cryopreservation and in vitro culture affect calcium signalling during human fertilization.Hum Reprod. 2014 Jan;29(1):29-40. doi: 10.1093/humrep/det404. Epub 2013 Nov 11. Hum Reprod. 2014. PMID: 24218403
-
A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Mol Hum Reprod. 2017 Sep 1;23(9):594-606. doi: 10.1093/molehr/gax032. Mol Hum Reprod. 2017. PMID: 28586460
-
Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization.Hum Reprod Update. 2015 Jul-Aug;21(4):427-54. doi: 10.1093/humupd/dmv011. Epub 2015 Mar 4. Hum Reprod Update. 2015. PMID: 25744083 Review.
-
Calcium signaling in oocyte quality and functionality and its application.Front Endocrinol (Lausanne). 2024 Aug 16;15:1411000. doi: 10.3389/fendo.2024.1411000. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39220364 Free PMC article. Review.
Cited by
-
Age-related calcium signaling disturbance restricted cAMP metabolism and induced ovarian oxidation stress in laying ducks.Poult Sci. 2025 Jan;104(1):104551. doi: 10.1016/j.psj.2024.104551. Epub 2024 Nov 22. Poult Sci. 2025. PMID: 39662254 Free PMC article.
-
Effects of meiotic stage-specific oocyte vitrification on mouse oocyte quality and developmental competence.Front Endocrinol (Lausanne). 2023 Jun 26;14:1200051. doi: 10.3389/fendo.2023.1200051. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37455899 Free PMC article.
-
In vivo and in vitro postovulatory aging: when time works against oocyte quality?J Assist Reprod Genet. 2022 Apr;39(4):905-918. doi: 10.1007/s10815-022-02418-y. Epub 2022 Mar 21. J Assist Reprod Genet. 2022. PMID: 35312936 Free PMC article. Review.
References
-
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324.e1–1331.e1. doi: 10.1016/j.fertnstert.2012.11.037. - DOI - PMC - PubMed
-
- Intracytoplasmic sperm injection (ICSI) for non-male factor infertility: a committee opinion. Fertility and sterility. 2012;98(6):1395–9. 10.1016/j.fertnstert.2012.08.026. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

