Risk-Based Monitoring in Clinical Trials: Past, Present, and Future
- PMID: 33914298
- PMCID: PMC8082746
- DOI: 10.1007/s43441-021-00295-8
Risk-Based Monitoring in Clinical Trials: Past, Present, and Future
Abstract
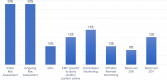
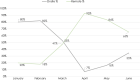
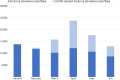
Risk-based monitoring (RBM) is a powerful tool for efficiently ensuring patient safety and data integrity in a clinical trial, enhancing overall trial quality. To better understand the state of RBM implementation across the clinical trial industry, the Association of Clinical Research Organizations (ACRO) conducted a landscape survey among its member companies across 6,513 clinical trials ongoing at the end of 2019. Of these trials, 22% included at least 1 of the 5 RBM components: key risk indicators (KRIs), centralized monitoring, off-site/remote-site monitoring, reduced source data verification (SDV), and reduced source document review (SDR). The implementation rates for the individual RBM components ranged 8%-19%, with the most frequently implemented component being centralized monitoring and the least frequently implemented being reduced SDR. When the COVID-19 pandemic emerged in early 2020, additional data were collected to assess its impact on trial monitoring, focusing specifically on trials switching from on-site monitoring to off-site/remote-site monitoring. These mid-pandemic data show that the vast majority of monitoring visits were on-site in February 2020, but an even higher percentage were off-site in April, corresponding with the first peak of the pandemic. Despite this shift, similar numbers of non-COVID-related protocol deviations were detected from February through June, suggesting little or no reduction in monitoring effectiveness. The pre- and mid-pandemic data provide two very different snapshots of RBM implementation, but both support the need to promote adoption of this approach while also highlighting an opportunity to capitalize on the recent shift toward greater RBM uptake in a post-pandemic environment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- ACRO. Establishing Risk-Based Monitoring within a Quality-Based System as “Best Practice” for Clinical Studies – ACRO [Internet]. 2019. https://www.acrohealth.org/rbqm-report/. Accessed 8 October 2020
-
- ACRO. Risk Based Quality Management (RBQM) – A Collaborative Approach to Holistic Clinical Trial Oversight [Internet]. 2019. https://www.acrohealth.org/oversight-rbqm-paper/. Accessed 8 October 2020
-
- EMA. Reflection paper on risk based quality management in clinical trials [Internet]. 2013. https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-p...
-
- ICH. Guideline for Good Clinical Practice E6(R2) [Internet]. 2016. https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf. Accessed 7 August 2020
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

