Inflammatory and Biomechanical Drivers of Endothelial-Interstitial Interactions in Calcific Aortic Valve Disease
- PMID: 33914601
- PMCID: PMC8519486
- DOI: 10.1161/CIRCRESAHA.121.318011
Inflammatory and Biomechanical Drivers of Endothelial-Interstitial Interactions in Calcific Aortic Valve Disease
Abstract
Calcific aortic valve disease is dramatically increasing in global burden, yet no therapy exists outside of prosthetic replacement. The increasing proportion of younger and more active patients mandates alternative therapies. Studies suggest a window of opportunity for biologically based diagnostics and therapeutics to alleviate or delay calcific aortic valve disease progression. Advancement, however, has been hampered by limited understanding of the complex mechanisms driving calcific aortic valve disease initiation and progression towards clinically relevant interventions.
Keywords: aortic valve; cardiovascular disease; cytokines; endothelial cells; inflammation.
Figures



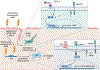


References
-
- Thubrikar M, Bosher LP, Nolan SP. The mechanism of opening of the aortic valve. J Thorac Cardiovasc Surg 1979;77:863–870. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

