Effects of exogenous ascorbic acid on seed germination and seedling salt-tolerance of alfalfa
- PMID: 33914821
- PMCID: PMC8084155
- DOI: 10.1371/journal.pone.0250926
Effects of exogenous ascorbic acid on seed germination and seedling salt-tolerance of alfalfa
Abstract
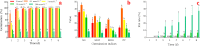
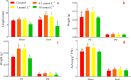
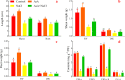
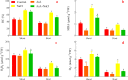
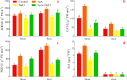
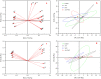
Alfalfa (Medicago sativa L.) is an important legume crop for forage, agriculture, and environment in the world. Ascorbic acid (AsA) plays positive roles in plants. However, its effects on germination and salt-tolerance of alfalfa are unknown. The effects of AsA applications on seed germination and seedling salt-tolerance of alfalfa were investigated. The results revealed that 0.1 and 1 mmol L-1 of exogenous AsA increased germination, amylase, and protease, as well as seedling length, fresh weight (FW), dry weight (DW), and endogenous AsA both in the shoots and roots, except that 1 mmol L-1 AsA reduced the activities of α-amylase, β-amylase and protease on day 3. However, 10 and 100 mmol L-1 AsA inhibited these parameters and even caused serious rot. It indicates that 0.1 mmol L-1 AsA has the optimal effects, whereas 100 mmol L-1 AsA has the worst impacts. Another part of the results showed that 0.1 mmol L-1 AsA not only enhanced stem elongation, FW and DW, but also increased chlorophyll and carotenoids both under non-stress and 150 mmol L-1 NaCl stress. Furthermore, 0.1 mmol L-1 AsA mitigated the damages of membrane permeability, malondialdehyde, and excessive reactive oxygen species (ROS) and ions both in the shoots and roots under 150 mmol L-1 NaCl stress. Hence, 0.1 mmol L-1 AsA improves growth and induces salt-tolerance by inhibiting excessive ROS, down-regulating the ion toxicity and up-regulating the antioxidant system. The principal component analysis included two main components both in the shoots and roots, and it explained the results well. In summary, the optimum concentration of 0.1 mmol L-1 AsA can be implemented to improve the seed germination and seedling growth of alfalfa under salt stress.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
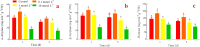
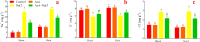
Similar articles
-
Salt-stress induced proteomic changes of two contrasting alfalfa cultivars during germination stage.J Sci Food Agric. 2019 Feb;99(3):1384-1396. doi: 10.1002/jsfa.9331. Epub 2018 Oct 25. J Sci Food Agric. 2019. PMID: 30144052
-
NaHS immersion alleviates the stress effect of chromium(III) on alfalfa seeds by affecting active oxygen metabolism.Plant Signal Behav. 2024 Dec 31;19(1):2375673. doi: 10.1080/15592324.2024.2375673. Epub 2024 Jul 7. Plant Signal Behav. 2024. PMID: 38972043 Free PMC article.
-
Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses.Plant Physiol Biochem. 2009 Jul;47(7):570-7. doi: 10.1016/j.plaphy.2009.02.009. Epub 2009 Feb 28. Plant Physiol Biochem. 2009. PMID: 19318268
-
Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance.Front Plant Sci. 2017 Apr 26;8:613. doi: 10.3389/fpls.2017.00613. eCollection 2017. Front Plant Sci. 2017. PMID: 28491070 Free PMC article. Review.
-
Physiological and biochemical roles of ascorbic acid on mitigation of abiotic stresses in plants.Plant Physiol Biochem. 2023 Sep;202:107970. doi: 10.1016/j.plaphy.2023.107970. Epub 2023 Aug 16. Plant Physiol Biochem. 2023. PMID: 37625254 Review.
Cited by
-
Dehydrin CaDHN2 Enhances Drought Tolerance by Affecting Ascorbic Acid Synthesis under Drought in Peppers.Plants (Basel). 2023 Nov 18;12(22):3895. doi: 10.3390/plants12223895. Plants (Basel). 2023. PMID: 38005792 Free PMC article.
-
Exogenous ascorbic acid as a potent regulator of antioxidants, osmo-protectants, and lipid peroxidation in pea under salt stress.BMC Plant Biol. 2024 Apr 5;24(1):247. doi: 10.1186/s12870-024-04947-3. BMC Plant Biol. 2024. PMID: 38575856 Free PMC article.
-
Biorefinery of sunflower by-products: Optimization of twin-screw extrusion for novel biostimulants.Heliyon. 2025 Feb 8;11(4):e42576. doi: 10.1016/j.heliyon.2025.e42576. eCollection 2025 Feb 28. Heliyon. 2025. PMID: 40028557 Free PMC article.
-
Transcriptome-Wide Analysis Revealed the Potential of the High-Affinity Potassium Transporter (HKT) Gene Family in Rice Salinity Tolerance via Ion Homeostasis.Bioengineering (Basel). 2022 Aug 23;9(9):410. doi: 10.3390/bioengineering9090410. Bioengineering (Basel). 2022. PMID: 36134956 Free PMC article.
-
Changes in Endogenous Carotenoids, Flavonoids, and Phenolics of Drought-Stressed Broccoli Seedlings After Ascorbic Acid Preconditioning.Plants (Basel). 2024 Dec 16;13(24):3513. doi: 10.3390/plants13243513. Plants (Basel). 2024. PMID: 39771211 Free PMC article.
References
-
- Wang XS, Han JG. 2007. Effects of NaCl and silicon on ion distribution in the roots, shoots and leaves of two alfalfa cultivars with different salt tolerance. Soil Sci Plant Nutr 53: 278–285. 10.1111/j.1747-0765.2007.00135.x. - DOI
-
- Jin JJ, Niu JP, Guo TT, Zhou RL, Sun LZ. 2020. The effect of drought on physiological responses of forage plants to salt stresses depends on occurring time. Acta Physiol Plant 42: 91. 10.1007/s11738-020-03083-3. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

