Drebrin attenuates atherosclerosis by limiting smooth muscle cell transdifferentiation
- PMID: 33914863
- PMCID: PMC8859638
- DOI: 10.1093/cvr/cvab156
Drebrin attenuates atherosclerosis by limiting smooth muscle cell transdifferentiation
Abstract
Aims: The F-actin-binding protein Drebrin inhibits smooth muscle cell (SMC) migration, proliferation, and pro-inflammatory signalling. Therefore, we tested the hypothesis that Drebrin constrains atherosclerosis.
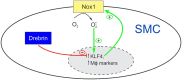
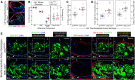
Methods and results: SM22-Cre+/Dbnflox/flox/Ldlr-/- (SMC-Dbn-/-/Ldlr-/-) and control mice (SM22-Cre+/Ldlr-/-, Dbnflox/flox/Ldlr-/-, and Ldlr-/-) were fed a western diet for 14-20 weeks. Brachiocephalic arteries of SMC-Dbn -/-/Ldlr-/- mice exhibited 1.5- or 1.8-fold greater cross-sectional lesion area than control mice at 14 or 20 weeks, respectively. Aortic atherosclerotic lesion surface area was 1.2-fold greater in SMC-Dbn-/-/Ldlr-/- mice. SMC-Dbn-/-/Ldlr-/- lesions comprised necrotic cores that were two-fold greater in size than those of control mice. Consistent with their bigger necrotic core size, lesions in SMC-Dbn-/- arteries also showed more transdifferentiation of SMCs to macrophage-like cells: 1.5- to 2.5-fold greater, assessed with BODIPY or with CD68, respectively. In vitro data were concordant: Dbn-/- SMCs had 1.7-fold higher levels of KLF4 and transdifferentiated to macrophage-like cells more readily than Dbnflox/flox SMCs upon cholesterol loading, as evidenced by greater up-regulation of CD68 and galectin-3. Adenovirally mediated Drebrin rescue produced equivalent levels of macrophage-like transdifferentiation in Dbn-/- and Dbnflox/flox SMCs. During early atherogenesis, SMC-Dbn-/-/Ldlr-/- aortas demonstrated 1.6-fold higher levels of reactive oxygen species than control mouse aortas. The 1.8-fold higher levels of Nox1 in Dbn-/- SMCs were reduced to WT levels with KLF4 silencing. Inhibition of Nox1 chemically or with siRNA produced equivalent levels of macrophage-like transdifferentiation in Dbn-/- and Dbnflox/flox SMCs.
Conclusion: We conclude that SMC Drebrin limits atherosclerosis by constraining SMC Nox1 activity and SMC transdifferentiation to macrophage-like cells.
Keywords: Atherosclerosis; Drebrin; Foam cell; NADPH oxidase; Nox1; Reactive oxygen species; VSMC; Vascular smooth muscle cells.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures





References
-
- Wang N, Tabas I, Winchester R, Ravalli S, Rabbani LE, Tall A.. Interleukin 8 is induced by cholesterol loading of macrophages and expressed by macrophage foam cells in human atheroma. J Biol Chem 1996;271:8837–8842. - PubMed
-
- Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, Fisher EA.. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol 2015;35:535–546. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

