Inflation-collapse dynamics drive patterning and morphogenesis in intestinal organoids
- PMID: 33915079
- PMCID: PMC8419000
- DOI: 10.1016/j.stem.2021.04.002
Inflation-collapse dynamics drive patterning and morphogenesis in intestinal organoids
Abstract
How stem cells self-organize to form structured tissues is an unsolved problem. Intestinal organoids offer a model of self-organization as they generate stem cell zones (SCZs) of typical size even without a spatially structured environment. Here we examine processes governing the size of SCZs. We improve the viability and homogeneity of intestinal organoid cultures to enable long-term time-lapse imaging of multiple organoids in parallel. We find that SCZs are shaped by fission events under strong control of ion channel-mediated inflation and mechanosensitive Piezo-family channels. Fission occurs through stereotyped modes of dynamic behavior that differ in their coordination of budding and differentiation. Imaging and single-cell transcriptomics show that inflation drives acute stem cell differentiation and induces a stretch-responsive cell state characterized by large transcriptional changes, including upregulation of Piezo1. Our results reveal an intrinsic capacity of the intestinal epithelium to self-organize by modulating and then responding to its mechanical state.
Keywords: intestinal stem cells; live imaging; mechanobiology; organoids; self-organization; size control; stretch-responsive cell.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.M.K. is a founder of 1CellBio, Inc.
Figures




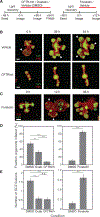
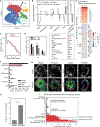
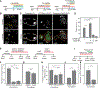
Comment in
-
Inflation comes before the fall: How epithelial stretch drives crypt fission.Cell Stem Cell. 2021 Sep 2;28(9):1505-1506. doi: 10.1016/j.stem.2021.08.008. Cell Stem Cell. 2021. PMID: 34478627
References
-
- Ayyaz A, Kumar S, Sangiorgi B, Ghoshal B, Gosio J, Ouladan S, Fink M, Barutcu S, Trcka D, Shen J, et al. (2019). Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569, 121–125. - PubMed
-
- Barker N (2014). Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol 15, 19–33. - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, and Clevers H (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

