Ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a systematic review and individual participant data meta-analysis
- PMID: 33915090
- PMCID: PMC8192305
- DOI: 10.1016/S2468-1253(21)00074-1
Ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a systematic review and individual participant data meta-analysis
Abstract
Background: Ursodeoxycholic acid is commonly used to treat intrahepatic cholestasis of pregnancy, yet its largest trial detected minimal benefit for a composite outcome (stillbirth, preterm birth, and neonatal unit admission). We aimed to examine whether ursodeoxycholic acid affects specific adverse perinatal outcomes.
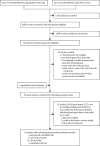
Methods: In this systematic review and individual participant data meta-analysis, we searched PubMed, Web of Science, Embase, MEDLINE, CINAHL, Global Health, MIDIRS, and Cochrane without language restrictions for relevant articles published between database inception, and Jan 1, 2020, using search terms referencing intrahepatic cholestasis of pregnancy, ursodeoxycholic acid, and perinatal outcomes. Eligible studies had 30 or more study participants and reported on at least one individual with intrahepatic cholestasis of pregnancy and bile acid concentrations of 40 μmol/L or more. We also included two unpublished cohort studies. Individual participant data were collected from the authors of selected studies. The primary outcome was the prevalence of stillbirth, for which we anticipated there would be insufficient data to achieve statistical power. Therefore, we included a composite of stillbirth and preterm birth as a main secondary outcome. A mixed-effects meta-analysis was done using multi-level modelling and adjusting for bile acid concentration, parity, and multifetal pregnancy. Individual participant data analyses were done for all studies and in different subgroups, which were produced by limiting analyses to randomised controlled trials only, singleton pregnancies only, or two-arm studies only. This study is registered with PROSPERO, CRD42019131495.
Findings: The authors of the 85 studies fulfilling our inclusion criteria were contacted. Individual participant data from 6974 women in 34 studies were included in the meta-analysis, of whom 4726 (67·8%) took ursodeoxycholic acid. Stillbirth occurred in 35 (0·7%) of 5097 fetuses among women with intrahepatic cholestasis of pregnancy treated with ursodeoxycholic acid and in 12 (0·6%) of 2038 fetuses among women with intrahepatic cholestasis of pregnancy not treated with ursodeoxycholic acid (adjusted odds ratio [aOR] 1·04, 95% CI 0·35-3·07; p=0·95). Ursodeoxycholic acid treatment also had no effect on the prevalence of stillbirth when considering only randomised controlled trials (aOR 0·29, 95% CI 0·04-2·42; p=0·25). Ursodeoxycholic acid treatment had no effect on the prevalence of the composite outcome in all studies (aOR 1·28, 95% CI 0·86-1·91; p=0·22), but was associated with a reduced composite outcome when considering only randomised controlled trials (0·60, 0·39-0·91; p=0·016).
Interpretation: Ursodeoxycholic acid treatment had no significant effect on the prevalence of stillbirth in women with intrahepatic cholestasis of pregnancy, but our analysis was probably limited by the low overall event rate. However, when considering only randomised controlled trials, ursodeoxycholic acid was associated with a reduction in stillbirth in combination with preterm birth, providing evidence for the clinical benefit of antenatal ursodeoxycholic acid treatment.
Funding: Tommy's, the Wellcome Trust, ICP Support, and the National Institute for Health Research.
Copyright © 2021 The Authors(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests CO and H-UM are consultants for Mirum Pharmaceuticals. CW is a consultant for Mirum Pharmaceuticals and GlaxoSmithKline. KK is an unpaid consultant for Myriad Pharmaceuticals. WMH reports non-financial support from the Falk Foundation, during the conduct of the study, and is co-author of the Cochrane review on pharmacological interventions for treating intrahepatic cholestasis of pregnancy.(14) RMT reports grants from Tommy's and the Lauren Page Trust during the conduct of the study. All other authors declare no competing interests.
Figures


Comment in
-
Ursodeoxycholic acid in intrahepatic cholestasis of pregnancy.Lancet Gastroenterol Hepatol. 2021 Jul;6(7):513-515. doi: 10.1016/S2468-1253(21)00099-6. Epub 2021 Apr 27. Lancet Gastroenterol Hepatol. 2021. PMID: 33915089 No abstract available.
-
Intrahepatic cholestasis of pregnancy: are we expecting too much from ursodeoxycholic acid?Lancet Gastroenterol Hepatol. 2021 Nov;6(11):886. doi: 10.1016/S2468-1253(21)00306-X. Lancet Gastroenterol Hepatol. 2021. PMID: 34626559 No abstract available.
-
Intrahepatic cholestasis of pregnancy: are we expecting too much from ursodeoxycholic acid? - Authors' reply.Lancet Gastroenterol Hepatol. 2021 Nov;6(11):886-887. doi: 10.1016/S2468-1253(21)00304-6. Lancet Gastroenterol Hepatol. 2021. PMID: 34626560 No abstract available.
References
-
- Bicocca MJ, Sperling JD, Chauhan SP. Intrahepatic cholestasis of pregnancy: review of six national and regional guidelines. Eur J Obstet Gynecol Reprod Biol. 2018;231:180–187. - PubMed
-
- Ovadia C, Williamson C. Intrahepatic cholestasis of pregnancy: recent advances. Clin Dermatol. 2016;34:327–334. - PubMed
-
- Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467–474. - PubMed
-
- Herrera CA, Manuck TA, Stoddard GJ. Perinatal outcomes associated with intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2018;31:1913–1920. - PubMed
Uncited References
-
- Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis of pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42:1399–1405. - PubMed
-
- Palma J, Reyes H, Ribalta J. Ursodeoxycholic acid in the treatment of cholestasis of pregnancy: a randomized double-blind study controlled with placebo. J Hepatol. 1997;27:1022–1028. - PubMed
-
- Ai Y, Liu SY. Comparison of three methods in the treatment of intrahepatic cholestasis of pregnancy. J Practical Obstet Gynecol. 2002;18:20–22.
-
- Floreani A, Paternoster D, Melis A, Grella PV. S-adenosylmethionine versus ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy: preliminary results of a controlled trial. Eur J Obstet Gynecol Repro Biol. 1996;67:109–113. - PubMed
-
- Kondrackiene J, Beuers U, Kupcinskas L. Efficacy and safety of ursodeoxycholic acid versus cholestyramine in intrahepatic cholestasis of pregnancy. Gastroenterology. 2005;129:894–901. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

