Psychedelic-inspired drug discovery using an engineered biosensor
- PMID: 33915107
- PMCID: PMC8122087
- DOI: 10.1016/j.cell.2021.03.043
Psychedelic-inspired drug discovery using an engineered biosensor
Abstract
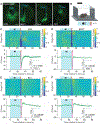
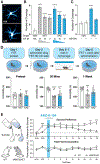
Ligands can induce G protein-coupled receptors (GPCRs) to adopt a myriad of conformations, many of which play critical roles in determining the activation of specific signaling cascades associated with distinct functional and behavioral consequences. For example, the 5-hydroxytryptamine 2A receptor (5-HT2AR) is the target of classic hallucinogens, atypical antipsychotics, and psychoplastogens. However, currently available methods are inadequate for directly assessing 5-HT2AR conformation both in vitro and in vivo. Here, we developed psychLight, a genetically encoded fluorescent sensor based on the 5-HT2AR structure. PsychLight detects behaviorally relevant serotonin release and correctly predicts the hallucinogenic behavioral effects of structurally similar 5-HT2AR ligands. We further used psychLight to identify a non-hallucinogenic psychedelic analog, which produced rapid-onset and long-lasting antidepressant-like effects after a single administration. The advent of psychLight will enable in vivo detection of serotonin dynamics, early identification of designer drugs of abuse, and the development of 5-HT2AR-dependent non-hallucinogenic therapeutics.
Keywords: depression; genetically encoded indicators; hallucinogen; psychedelic; serotonin; serotonin receptors.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.E.O. is a co-founder of Delix Therapeutics. L.T. is a co-founder of Seven Biosciences. C.L. was a part-time employee of Seven Biosciences. Delix Therapeutics and Seven Biosciences have licensed technology from the University of California, Davis related to this manuscript. All other authors declare no competing interests.
Figures




References
-
- Aghajanian GK, and Marek GJ (1999). Serotonin and Hallucinogens. Neuropsychopharmacology 21, 16–23. - PubMed
-
- Benes H, Deissler A, Rodenbeck A, Engfer A, and Kohnen R (2006). Lisuride treatment of restless legs syndrome: first studies with monotherapy in de novo patients and in combination with levodopa in advanced disease. J Neural Transm (Vienna) 113, 87–92. - PubMed
-
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, and Clarke WP (1998). Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol 54, 94–104. - PubMed
-
- Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Cumbay MG, Watts VJ, Barker EL, and Nichols DE (2000). Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J Med Chem 43, 4701–4710. - PubMed
-
- Broussard GJ, Unger EK, Liang R, McGrew BP, and Tian L (2018). Imaging Glutamate with Genetically Encoded Fluorescent Sensors. In Biochemical Approaches for Glutamatergic Neurotransmission, Parrot S, and Denoroy L, eds. (New York, NY: Springer New York; ), pp. 117–153.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

