SARS-CoV-2 serology testing: Progress and challenges
- PMID: 33915155
- PMCID: PMC8071778
- DOI: 10.1016/j.jim.2021.113060
SARS-CoV-2 serology testing: Progress and challenges
Abstract
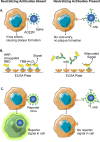
The coronavirus disease 2019 (COVID-19) pandemic has caused the most devasting social and economic impact of this century. The current pandemic will end only after a safe, effective vaccine becomes available and protective herd immunity has been achieved through vaccination. The key parameter to gauge protective immunity is neutralizing antibody levels. Thus, reliable serology testing is essential to diagnose whether an individual has been previously infected, as a large proportion of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections is asymptomatic. For both naturally infected and vaccinated individuals, it is critical to monitor their neutralizing antibody titers over time. This is because, when neutralizing antibody levels wane below a threshold which remains to be determined, they become vulnerable to reinfection. Due to the importance of serology testing, academia and industry have developed different platforms for serological diagnosis, many of which have achieved the Food and Drug Administration (FDA) Emergency Use Authorizations (EUA). Here we summarize the status of COVID-19 serology testing, discuss challenges, and provide future directions for improvement.
Keywords: Binding antibodies; COVID-19; Neutralizing antibodies; SARS-CoV-2; Serology testing.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures
References
-
- Almahboub S.A., Algaissi A., Alfaleh M.A., ElAssouli M.Z., Hashem A.M. Evaluation of neutralizing antibodies against highly pathogenic coronaviruses: a detailed protocol for a rapid evaluation of neutralizing antibodies using vesicular stomatitis virus pseudovirus-based assay. Front Microbiol. 2020:11. doi: 10.3389/fmicb.2020.02020. - DOI - PMC - PubMed
-
- Amanat F., White K.M., Miorin L., Strohmeier S., McMahon M., Meade P., Liu W.-C., Albrecht R.A., Simon V., Martinez-Sobrido L., Moran T., Garcia-Sastre A., Krammer F. An in vitro microneutralization assay for SARS-CoV-2 serology and drug screening. Curr Protoc Microbiol. 2020;e108:58. doi: 10.1002/cpmc.108. - DOI - PMC - PubMed
-
- Case J.B., Rothlauf P.W., Chen R.E., Liu Z., Zhao H., Kim A.S., Bloyet L.-M., Zeng Q., Tahan S., Droit L., Ilagan M.X.G., Tartell M.A., Amarasinghe G., Henderson J.P., Miersch S., Ustav M., Sidhu S., Virgin H.W., Wang D., Ding S., Corti D., Theel E.S., Fremont D.H., Diamond M.S., Whelan S.P.J. Neutralizing antibody and soluble ACE2 inhibition of a replication-competent VSV-SARS-CoV-2 and a clinical isolate of SARS-CoV-2. Cell Host Microbe. 2020;28:475–485. doi: 10.1016/j.chom.2020.06.021. - DOI - PMC - PubMed
-
- Charlton C.L., Kanji J.N., Johal K., Bailey A., Plitt S.S., MacDonald C., Kunst A., Buss E., Burnes L.E., Fonseca K., Berenger B.M., Schnabl K., Hu J., Stokes W., Zelyas N., Tipples G. Evaluation of six commercial mid- to high-volume antibody and six point-of-care lateral flow assays for detection of SARS-CoV-2 antibodies. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01361-20. e01361–20. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

