Heparin: A simplistic repurposing to prevent SARS-CoV-2 transmission in light of its in-vitro nanomolar efficacy
- PMID: 33915212
- PMCID: PMC8074525
- DOI: 10.1016/j.ijbiomac.2021.04.148
Heparin: A simplistic repurposing to prevent SARS-CoV-2 transmission in light of its in-vitro nanomolar efficacy
Abstract
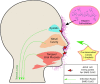
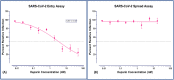
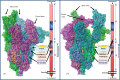
The world is currently facing a novel coronavirus (SARS-CoV-2) pandemic. The greatest threat that is disrupting the normal functioning of society is the exceptionally high species independent transmission. Drug repurposing is understood to be the best strategy to immediately deploy well-characterized agents against new pathogens. Several repurposable drugs are already in evaluation for determining suitability to treat COVID-19. One such promising compound includes heparin, which is widely used in reducing thrombotic events associated with COVID-19 induced pathology. As part of identifying target-specific antiviral compounds among FDA and world-approved libraries using high-throughput virtual screening (HTVS), we previously evaluated top hits for anti-SARS-CoV-2 activity. Here, we report results of highly efficacious viral entry blocking properties of heparin (IC50 = 12.3 nM) in the complete virus assay, and further, propose ways to use it as a potential transmission blocker. Exploring further, our in-silico analysis indicated that the heparin interacts with post-translational glycoconjugates present on spike proteins. The patterns of accessible spike-glycoconjugates in open and closed states are completely contrasted by one another. Heparin-binding to the open conformation of spike structurally supports the state and may aid ACE2 binding as reported with cell surface-bound heparan sulfate. We also studied spike protein mutant variants' heparin interactions for possible resistance. Based on available data and optimal absorption properties by the skin, heparin could potentially be used to block SARS-CoV-2 transmission. Studies should be designed to exploit its nanomolar antiviral activity to formulate heparin as topical or inhalation-based formulations, particularly on exposed areas and sites of primary viremia e.g. ACE2 rich epithelia of the eye (conjunctiva/lids), nasal cavity, and mouth.
Keywords: Anticoagulant; COVID-19; Heparin; Lipid-based formulations; SARS-CoV2; Spike protein; Sulfated polysaccharide; Topical delivery; Transmission-blocking.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Computational repurposing approach for targeting the critical spike mutations in B.1.617.2 (delta), AY.1 (delta plus) and C.37 (lambda) SARS-CoV-2 variants using exhaustive structure-based virtual screening, molecular dynamic simulations and MM-PBSA methods.Comput Biol Med. 2022 Aug;147:105709. doi: 10.1016/j.compbiomed.2022.105709. Epub 2022 Jun 7. Comput Biol Med. 2022. PMID: 35728285 Free PMC article.
-
Identification of SARS-CoV-2 Receptor Binding Inhibitors by In Vitro Screening of Drug Libraries.Molecules. 2021 May 27;26(11):3213. doi: 10.3390/molecules26113213. Molecules. 2021. PMID: 34072087 Free PMC article.
-
Different compounds against Angiotensin-Converting Enzyme 2 (ACE2) receptor potentially containing the infectivity of SARS-CoV-2: an in silico study.J Mol Model. 2022 Mar 5;28(4):82. doi: 10.1007/s00894-022-05059-1. J Mol Model. 2022. PMID: 35249180 Free PMC article.
-
Structure based Drug Designing Approaches in SARS-CoV-2 Spike Inhibitor Design.Curr Top Med Chem. 2022;22(29):2396-2409. doi: 10.2174/1568026623666221103091658. Curr Top Med Chem. 2022. PMID: 36330617 Review.
-
COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics.Infection. 2021 Apr;49(2):199-213. doi: 10.1007/s15010-020-01516-2. Epub 2020 Sep 4. Infection. 2021. PMID: 32886331 Free PMC article.
Cited by
-
Is heparan sulfate a target for inhibition of RNA virus infection?Am J Physiol Cell Physiol. 2022 Apr 1;322(4):C605-C613. doi: 10.1152/ajpcell.00028.2022. Epub 2022 Feb 23. Am J Physiol Cell Physiol. 2022. PMID: 35196165 Free PMC article. Review.
-
Iron dysregulation in COVID-19 and reciprocal evolution of SARS-CoV-2: Natura nihil frustra facit.J Cell Biochem. 2022 Mar;123(3):601-619. doi: 10.1002/jcb.30207. Epub 2022 Jan 8. J Cell Biochem. 2022. PMID: 34997606 Free PMC article.
-
An engineered recombinant protein containing three structural domains in SARS-CoV-2 S2 protein has potential to act as a pan-human coronavirus entry inhibitor or vaccine antigen.Emerg Microbes Infect. 2023 Dec;12(2):2244084. doi: 10.1080/22221751.2023.2244084. Emerg Microbes Infect. 2023. PMID: 37534910 Free PMC article.
-
Structural and kinetic analyses of holothurian sulfated glycans suggest potential treatment for SARS-CoV-2 infection.J Biol Chem. 2021 Oct;297(4):101207. doi: 10.1016/j.jbc.2021.101207. Epub 2021 Sep 17. J Biol Chem. 2021. PMID: 34537241 Free PMC article.
-
Evaluation interaction of graphene oxide with heparin for antiviral blockade: a study of ab initio simulations, molecular docking, and experimental analysis.J Mol Model. 2023 Jul 7;29(8):235. doi: 10.1007/s00894-023-05645-x. J Mol Model. 2023. PMID: 37418181
References
-
- Organization WH, et al. 2019. The Selection and Use of Essential Medicines: Report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2019 (Including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for Children)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

