Longitudinal infant fNIRS channel-space analyses are robust to variability parameters at the group-level: An image reconstruction investigation
- PMID: 33915275
- PMCID: PMC8285580
- DOI: 10.1016/j.neuroimage.2021.118068
Longitudinal infant fNIRS channel-space analyses are robust to variability parameters at the group-level: An image reconstruction investigation
Abstract
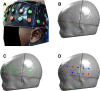
The first 1000 days from conception to two-years of age are a critical period in brain development, and there is an increasing drive for developing technologies to help advance our understanding of neurodevelopmental processes during this time. Functional near-infrared spectroscopy (fNIRS) has enabled longitudinal infant brain function to be studied in a multitude of settings. Conventional fNIRS analyses tend to occur in the channel-space, where data from equivalent channels across individuals are combined, which implicitly assumes that head size and source-detector positions (i.e. array position) on the scalp are constant across individuals. The validity of such assumptions in longitudinal infant fNIRS analyses, where head growth is most rapid, has not previously been investigated. We employed an image reconstruction approach to analyse fNIRS data collected from a longitudinal cohort of infants in The Gambia aged 5- to 12-months. This enabled us to investigate the effect of variability in both head size and array position on the anatomical and statistical inferences drawn from the data at both the group- and the individual-level. We also sought to investigate the impact of group size on inferences drawn from the data. We found that variability in array position was the driving factor between differing inferences drawn from the data at both the individual- and group-level, but its effect was weakened as group size increased towards the full cohort size (N = 53 at 5-months, N = 40 at 8-months and N = 45 at 12-months). We conclude that, at the group sizes in our dataset, group-level channel-space analysis of longitudinal infant fNIRS data is robust to assumptions about head size and array position given the variability in these parameters in our dataset. These findings support a more widespread use of image reconstruction techniques in longitudinal infant fNIRS studies.
Keywords: Functional near-infrared spectroscopy; Image reconstruction; Infant cognitive development; Infant functional neuroimaging; Longitudinal imaging; Neurodevelopment.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest R.J.C. has financial interests in Gowerlabs Ltd, a manufacturer of fNIRS technologies.
Figures












References
-
- Arridge, S., & Cooper, R. J. (2015). Optical Image Reconstruction. In A. W. Toga (Ed.), Brain Mapping: an Encyclopedic Reference (1, pp. 217–222). Elsevier Science. https://doi.org/10.1016/B978-0-12-397025-1.00288-8
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

