Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis, first update
- PMID: 33915284
- PMCID: PMC8076756
- DOI: 10.1016/j.cmi.2021.04.019
Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis, first update
Abstract
Objectives: Cytokine release syndrome with elevated interleukin-6 (IL-6) levels is associated with multiorgan damage and death in severe coronavirus disease 2019 (COVID-19). Our objective was to update the data in a living systematic review of the literature concerning the efficacy and toxicity of the IL-6 receptor antagonist tocilizumab in COVID-19 patients.
Methods: Data sources were Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, Scopus up, preprint servers and Google from 8th October 2020 till 24th February 2021. Eligible studies were randomized controlled trials (RCTs) and observational studies at low or moderate risk of bias. The participants were hospitalized COVID-19 patients, and intervention was tocilizumab versus placebo or standard of care. We pooled crude risk ratios (RRs) of RCTs with a random effects model and evaluated inconsistency between studies with I2. We assessed the certainty of evidence using the GRADE approach.
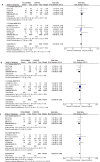
Results: Of 1600 citations, eight RCTs and 28 cohorts were eligible. The eight RCTs had low risk of bias, and with 6311 patients they examined the effect of tocilizumab on short-term mortality; pooled RR was 0.91 (95%CI 0.78-1.07, I2 25%). Only the REMAP-CAP and RECOVERY trials, with the majority of their patients on concomitant corticosteroids, showed lower 30-day mortality with tocilizumab use: RR 0.74 (95%CI 0.59-0.93) and 0.89 (95%CI 0.81-0.97), respectively. Seven RCTs, with 5391 patients, examined the effect of tocilizumab on risk of mechanical ventilation; pooled RR was 0.84 (95%CI 0.76-0.93), I2 0%, with a corresponding number needed to treat of 20 (95%CI 14.3-33.3). Eight RCTs, with 5340 patients, examined the effect of tocilizumab on a composite of poor outcome; pooled RR was 0.82 (95%CI 0.76-0.90, I2 3%). Data from the RCTs showed a lower risk of infections and no higher risk of serious adverse events with tocilizumab: pooled RR 0.67 (95%CI 0.45-0.99, eight RCTs) and 0.85 (95%CI 0.63-1.16, seven RCTs), respectively. Among 28 cohorts with 15 484 patients, the pooled adjusted RR for mortality was 0.53 (95%CI 0.43-0.67, I2 76%).
Conclusions: Cumulative high-certainty evidence shows that tocilizumab reduces the risk of mechanical ventilation in hospitalized patients with severe COVID-19. Moderate-certainty evidence shows that tocilizumab reduces the risk of poor outcome and the risk of secondary infections in hospitalized COVID-19 patients. This review will continuously evaluate the role of tocilizumab in COVID-19 treatment.
Keywords: COVID; Meta-analysis; Outcome; Systematic review; Tocilizumab.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Re: 'Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis' by Tleyjeh et al.Clin Microbiol Infect. 2021 Aug;27(8):1175-1176. doi: 10.1016/j.cmi.2021.01.025. Epub 2021 Feb 5. Clin Microbiol Infect. 2021. PMID: 33549765 Free PMC article. No abstract available.
-
Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis - Author's reply.Clin Microbiol Infect. 2021 Aug;27(8):1177-1178. doi: 10.1016/j.cmi.2021.02.024. Epub 2021 Mar 8. Clin Microbiol Infect. 2021. PMID: 33705848 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

