Germinated Rhynchosia nulubilis Fermented with Lactobacillus pentosus SC65 Reduces Particulate Matter Induced Type II Alveolar Epithelial Apoptotic Cell Death
- PMID: 33915904
- PMCID: PMC8038076
- DOI: 10.3390/ijms22073660
Germinated Rhynchosia nulubilis Fermented with Lactobacillus pentosus SC65 Reduces Particulate Matter Induced Type II Alveolar Epithelial Apoptotic Cell Death
Abstract
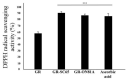
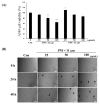
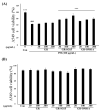
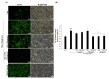
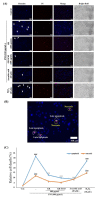
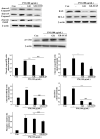
Particulate matter (PM) is a significant environmental pollutant that promotes respiratory diseases, including lung injury and inflammation, by inducing oxidative stress. Rhynchosia nulubilis (black soybean) is traditionally used to prevent chronic respiratory disease via inducing antioxidant and anti-inflammatory effects. To investigate the effects of Lactobacillus pentosus SC65 fermented GR (GR-SC65) and Pediococcus pentosaceus ON81A (GR-ON81A) against PM-induced oxidative stress and cell death in A549 cells, we performed the 2-7-dichlorodihydrofluorescein diacetate and cell counting kit-8 assays, as well as Hoechst 33342 and propidium iodide staining and western blotting. GR-SC65 showed the highest total polyphenolic contents and 1,1-diphenyl-2-picrylidrazil radical scavenging activity among lactic acid bacteria-fermented GRs (p < 0.001 vs. GR). Four soy peptides, β-conglycinin breakdowns (INAENNQRNF, ISSEDKPFN, LAFPGSAQAVEK, and LAFPGSAKDIEN), were detected in GR-SC65, but not in GR. In GR-SC65, PM-induced A549 cell death was less than that observed in GR-ON81A and GR (p < 0.001 vs. PM-treated group). GR-SC65 significantly decreased intracellular reactive oxidative species (ROS) when compared with PM (*** p < 0.001 vs. PM). GR-SC65 decreased the levels of BAX, active caspase-9, -3, and poly ADP-ribose polymerase (PARP) proteins (#p < 0.01, ###p < 0.001 vs. PM), while increasing the level of BCL-2 protein, a mitochondrial anti-apoptotic protein (###p < 0.001 vs. PM). Our findings indicate that GR-SC65 inhibited PM-induced cell death by suppressing the levels of ROS, active caspase-9 and -3, and PARP proteins, while enhancing the level of BCL-2 protein in type II alveolar epithelial A549 cells. Therefore, GR-SC65 might be a potential therapeutic and preventive agent against PM-induced lung injury.
Keywords: A549 cells; PM < 11; ROS; apoptosis; cell death; germinated Rhynchosia nulubilis fermented with Lactobacillus pentosus SC65.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the study design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Chitosan Nanoparticle-Encapsulated Cordyceps militaris Grown on Germinated Rhynchosia nulubilis Reduces Type II Alveolar Epithelial Cell Apoptosis in PM2.5-Induced Lung Injury.Int J Mol Sci. 2025 Jan 27;26(3):1105. doi: 10.3390/ijms26031105. Int J Mol Sci. 2025. PMID: 39940873 Free PMC article.
-
Cordyceps militaris Grown on Germinated Rhynchosia nulubilis (GRC) Encapsulated in Chitosan Nanoparticle (GCN) Suppresses Particulate Matter (PM)-Induced Lung Inflammation in Mice.Int J Mol Sci. 2024 Oct 3;25(19):10642. doi: 10.3390/ijms251910642. Int J Mol Sci. 2024. PMID: 39408971 Free PMC article.
-
Germinated black soybean fermented with Lactobacillus pentosus SC65 alleviates DNFB-induced delayed-type hypersensitivity in C57BL/6N mice.J Ethnopharmacol. 2021 Jan 30;265:113236. doi: 10.1016/j.jep.2020.113236. Epub 2020 Aug 1. J Ethnopharmacol. 2021. PMID: 32750462
-
Inhibition of inflammation-induced injury and cell migration by coelonin and militarine in PM2.5-exposed human lung alveolar epithelial A549 cells.Eur J Pharmacol. 2021 Apr 5;896:173931. doi: 10.1016/j.ejphar.2021.173931. Epub 2021 Feb 4. Eur J Pharmacol. 2021. PMID: 33549578
-
Inflammatory cell signaling following exposures to particulate matter and ozone.Biochim Biophys Acta. 2016 Dec;1860(12):2826-34. doi: 10.1016/j.bbagen.2016.03.030. Epub 2016 Mar 23. Biochim Biophys Acta. 2016. PMID: 27015762 Review.
Cited by
-
Lactic Acid Bacteria Fermented Cordyceps militaris (GRC-SC11) Suppresses IgE Mediated Mast Cell Activation and Type I Hypersensitive Allergic Murine Model.Nutrients. 2021 Oct 28;13(11):3849. doi: 10.3390/nu13113849. Nutrients. 2021. PMID: 34836105 Free PMC article.
-
Chitosan Nanoparticle-Encapsulated Cordyceps militaris Grown on Germinated Rhynchosia nulubilis Reduces Type II Alveolar Epithelial Cell Apoptosis in PM2.5-Induced Lung Injury.Int J Mol Sci. 2025 Jan 27;26(3):1105. doi: 10.3390/ijms26031105. Int J Mol Sci. 2025. PMID: 39940873 Free PMC article.
-
Negative Feedback of the cAMP/PKA Pathway Regulates the Effects of Endoplasmic Reticulum Stress-Induced NLRP3 Inflammasome Activation on Type II Alveolar Epithelial Cell Pyroptosis as a Novel Mechanism of BLM-Induced Pulmonary Fibrosis.J Immunol Res. 2022 Aug 18;2022:2291877. doi: 10.1155/2022/2291877. eCollection 2022. J Immunol Res. 2022. PMID: 36033388 Free PMC article.
-
Therapeutic Potential of Herbal Medicines in Combating Particulate Matter (PM)-Induced Health Effects: Insights from Recent Studies.Antioxidants (Basel). 2024 Dec 27;14(1):23. doi: 10.3390/antiox14010023. Antioxidants (Basel). 2024. PMID: 39857357 Free PMC article. Review.
-
Cordyceps militaris Grown on Germinated Rhynchosia nulubilis (GRC) Encapsulated in Chitosan Nanoparticle (GCN) Suppresses Particulate Matter (PM)-Induced Lung Inflammation in Mice.Int J Mol Sci. 2024 Oct 3;25(19):10642. doi: 10.3390/ijms251910642. Int J Mol Sci. 2024. PMID: 39408971 Free PMC article.
References
-
- Cui Y., Xie X., Jia F., He J., Li Z., Fu M., Hao H., Liu Y., Liu J.Z., Cowan P.J., et al. Ambient Fine Particulate Matter Induces Apoptosis of Endothelial Progenitor Cells Through Reactive Oxygen Species Formation. Cell. Physiol. Biochem. 2015;35:353–363. doi: 10.1159/000369701. - DOI - PMC - PubMed
-
- Fernando I.S., Jayawardena T.U., Kim H.-S., Lee W.W., Vaas A., De Silva H., Abayaweera G., Nanayakkara C., Abeytunga D., Lee D.-S., et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh) Environ. Res. 2019;172:150–158. doi: 10.1016/j.envres.2019.02.016. - DOI - PubMed
-
- Hiura T.S., Kaszubowski M.P., Li N., Nel A.E. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J. Immunol. 1999;163:5582–5591. - PubMed
-
- Wei H., Liang F., Cheng W., Zhou R., Wu X., Feng Y., Wang Y. The mechanisms for lung cancer risk of PM2.5: Induction of epithelial-mesenchymal transition and cancer stem cell properties in human non-small cell lung cancer cells. Environ. Toxicol. 2017;32:2341–2351. doi: 10.1002/tox.22437. - DOI - PubMed
-
- Billet S., Garçon G., Dagher Z., Verdin A., LeDoux F., Cazier F., Courcot D., Aboukais A., Shirali P. Ambient particulate matter (PM2.5): Physicochemical characterization and metabolic activation of the organic fraction in human lung epithelial cells (A549) Environ. Res. 2007;105:212–223. doi: 10.1016/j.envres.2007.03.001. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

