Exosomes from Plasma of Neuroblastoma Patients Contain Doublestranded DNA Reflecting the Mutational Status of Parental Tumor Cells
- PMID: 33915956
- PMCID: PMC8036333
- DOI: 10.3390/ijms22073667
Exosomes from Plasma of Neuroblastoma Patients Contain Doublestranded DNA Reflecting the Mutational Status of Parental Tumor Cells
Abstract
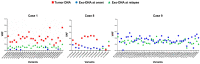
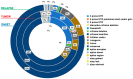
Neuroblastoma (NB) is an aggressive infancy tumor, leading cause of death among preschool age diseases. Here we focused on characterization of exosomal DNA (exo-DNA) isolated from plasma cell-derived exosomes of neuroblastoma patients, and its potential use for detection of somatic mutations present in the parental tumor cells. Exosomes are small extracellular membrane vesicles secreted by most cells, playing an important role in intercellular communications. Using an enzymatic method, we provided evidence for the presence of double-stranded DNA in the NB exosomes. Moreover, by whole exome sequencing, we demonstrated that NB exo-DNA represents the entire exome and that it carries tumor-specific genetic mutations, including those occurring on known oncogenes and tumor suppressor genes in neuroblastoma (ALK, CHD5, SHANK2, PHOX2B, TERT, FGFR1, and BRAF). NB exo-DNA can be useful to identify variants responsible for acquired resistance, such as mutations of ALK, TP53, and RAS/MAPK genes that appear in relapsed patients. The possibility to isolate and to enrich NB derived exosomes from plasma using surface markers, and the quick and easy extraction of exo-DNA, gives this methodology a translational potential in the clinic. Exo-DNA can be an attractive non-invasive biomarker for NB molecular diagnostic, especially when tissue biopsy cannot be easily available.
Keywords: ALK; exo-DNA; exosomes; genotypability; neuroblastoma; tumor mutation load.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
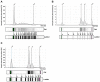

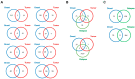

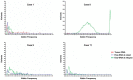
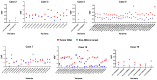
References
-
- Cohn S.L., Pearson A.D., London W.B., Monclair T., Ambros P.F., Brodeur G.M., Faldum A., Hero B., Iehara T., Machin D., et al. INRG Task Force. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J. Clin. Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

