Pharmacokinetic Changes According to Single or Multiple Oral Administrations of Socheongryong-Tang to Rats: Presented as a Typical Example of Changes in the Pharmacokinetics Following Multiple Exposures to Herbal Medicines
- PMID: 33916059
- PMCID: PMC8103508
- DOI: 10.3390/pharmaceutics13040478
Pharmacokinetic Changes According to Single or Multiple Oral Administrations of Socheongryong-Tang to Rats: Presented as a Typical Example of Changes in the Pharmacokinetics Following Multiple Exposures to Herbal Medicines
Abstract
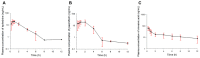
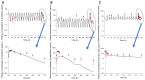
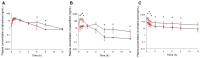
The purpose of this study was to investigate the pharmacokinetic properties of ephedrine, paeoniflorin, and cinnamic acid after single or multiple doses of Socheongryong-tang (SCRT) were administered to rats, and to present an example of the pharmacokinetic changes following multiple doses of an herbal medicine. SCRT is a traditional herbal medicine that has been used clinically for a long time, and its main ingredients include ephedrine, paeoniflorin, and cinnamic acid. However, studies on the pharmacokinetic properties of SCRT are insufficient, and particularly, no pharmacokinetic information has been reported for multiple doses. In this study, SCRT was administered orally to rats once or multiple times, and plasma sampled at different times was quantitatively analyzed for ephedrine, paeoniflorin, and cinnamic acid using ultra-high-performance liquid chromatography-tandem mass spectrometry. There was a difference between the pharmacokinetic parameter values of each component (especially in paeoniflorin and cinnamic acid) obtained after single or multiple doses of SCRT. The actual observed values of each component obtained after multiple doses of SCRT were clearly different from the predicted results of multiple-dose simulations based on the pharmacokinetic profiles obtained after a single dose. The results confirmed that the plasma concentrations and, thus, exposures to paeoniflorin and cinnamic acid were significantly increased when SCRT was administered multiple times, whereas that of ephedrine was not. The results of this study are expected to provide useful pharmacokinetic data for the safety and efficacy evaluation of SCRT in the future and demonstrate the necessity of pharmacokinetic comparison studies according to single or multiple oral administrations of herbal medicines.
Keywords: Socheongryong-tang; cinnamic acid; ephedrine; paeoniflorin; pharmacokinetic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Simultaneous UPLC-MS/MS determination of four components of Socheongryong-tang tablet in human plasma: Application to pharmacokinetic study.J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Sep 15;1095:214-225. doi: 10.1016/j.jchromb.2018.07.043. Epub 2018 Jul 31. J Chromatogr B Analyt Technol Biomed Life Sci. 2018. PMID: 30081350
-
Expression of Cytochrome P450s in the Liver of Rats Administered with Socheongryong-tang, a Traditional Herbal Formula.Pharmacogn Mag. 2016 Jul-Sep;12(47):211-8. doi: 10.4103/0973-1296.186340. Pharmacogn Mag. 2016. PMID: 27601852 Free PMC article.
-
Food- and gender-dependent pharmacokinetics of paeoniflorin after oral administration with Samul-tang in rats.J Ethnopharmacol. 2012 Jun 26;142(1):161-7. doi: 10.1016/j.jep.2012.04.032. Epub 2012 Apr 21. J Ethnopharmacol. 2012. PMID: 22543167
-
Simultaneous determination of calycosin-7-O-β-D-glucoside, cinnamic acid, paeoniflorin and albiflorin in rat plasma by UHPLC-MS/MS and its application to a pharmacokinetic study of Huangqi Guizhi Wuwu Decoction.J Pharm Biomed Anal. 2019 Jun 5;170:1-7. doi: 10.1016/j.jpba.2019.03.022. Epub 2019 Mar 14. J Pharm Biomed Anal. 2019. PMID: 30897430
-
Determination the active compounds of herbal preparation by UHPLC-MS/MS and its application on the preclinical pharmacokinetics of pure ephedrine, single herbal extract of Ephedra, and a multiple herbal preparation in rats.J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Jul 15;1026:152-161. doi: 10.1016/j.jchromb.2015.12.027. Epub 2015 Dec 18. J Chromatogr B Analyt Technol Biomed Life Sci. 2016. PMID: 26724253
Cited by
-
Rutin and Physalis peruviana Extract: Population Pharmacokinetics in New Zealand Rabbits.Pharmaceutics. 2024 Sep 24;16(10):1241. doi: 10.3390/pharmaceutics16101241. Pharmaceutics. 2024. PMID: 39458573 Free PMC article.
References
-
- Kim Y.-E., Son M.J., Jung S.Y., Kwon O., Lee J.-H., Lee D.-H. Socheongryong-tang for improving nasal symptoms associated with allergic rhinitis: A study protocol for a randomized, open-label, cetirizine controlled, clinical trial. Medicine. 2018;97:e11812. doi: 10.1097/MD.0000000000011812. - DOI - PMC - PubMed
-
- Bae J.-S., Kim I.-S., Seo B.-D., Kim B.-J. Effects of Socheongryong-Tang, a Traditional Chinese Medicine, on Gastrointestinal Motility Disorders (Diabetic Models) in Mice. J. Korean Med. Obes. Res. 2017;17:61–67.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

